The idea of artificial perfusion goes back to the French physiologist Jean-Jacques Le Gallois, who perfused the heads of decapitated rabbits to prove that blood circulation maintained organ function [7]. The prototype of the temperature-controlled heart-lung machine was designed by von Frey and Gruber in 1884 [10], but it was Hooker who built the precursor of the film oxygenator in 1915: this consisted of a rubber disc over which the blood spread in an oxygenated film by direct contact with a stream of O2 [5]. These devices were not very successful because the blood coagulated very quickly. It was not until the discovery of heparin in 1916 and protamine 20 years later that this problem was solved [9]. It wasn't until 1937 that John Gibbon created the first complete extracorporeal circulation (ECC) machine to allow laboratory animals to survive; the oxygenator was a rotating disc screen [3].
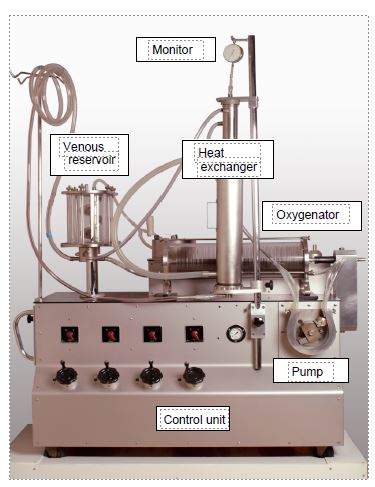
On 5 April 1951, Dennis used a ECC machine for the first time in a clinic in Minneapolis to correct an atrio-ventricular canal in a 6-year-old child; it was a surgical failure [1]. The following year, the second attempt (to close an ASD) also ended in the child's death. But on 20 May 1953, in Philadelphia, Gibbon successfully closed an ASD in an 18-year-old patient during a 45-minute bypass operation [4]. For Gibbon, this was the culmination of 23 years of research and design of extracorporeal circuits. He became the "father of ECC" and the first perfusionist in history. The first ECC circuits were cumbersome and dangerous; they required the priming of several litres of blood and worked with an oxygenator consisting of large discs partially immersed in the blood and rotating in a chamber full of oxygen. Everything was washed and reused for another patient. Only the tubes were disposable. This was the case with the first machine in Lausanne, the Livio-Mettraux machine of 1960 (Figure 1.2).
Figure 1.2: Livio-Mettraux bypass machine, built in Lausanne in 1960 and used in clinics until 1966. It already contained all the components of a modern machine: venous reservoir, roller pump, oxygenator, heat exchanger and monitoring. Only the tubing was single-use; the venous reservoir, oxygenator and heat exchanger were reusable systems. The oxygenator is a disc model: a series of vertical discs rotate on a slightly inclined axis and plunge into the blood flowing at the bottom of the cylindrical tank; the blood carried to the surface of the discs is thus exposed to a stream of oxygen which passes through the cylinder. The pump is a roller pump very similar to those used on today's circuits. The only monitor is a pressure gauge on the arterial circuit.
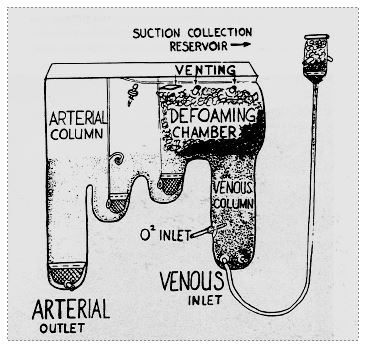
Figure 1.3: Bubble oxygenator by Ryggand Kyvsgaard (1956). Venous blood is oxygenated by simple bubbling. A chamber filled with antifoam performs debubbling. A cascade of three tanks completes the elimination of bubbles and allows volume to be stocked [8].
The problem of the oxygenator remained a nagging one, as the disc models caused countless problems and were inefficient. As early as 1956, DeWall and Lillehei designed a system that could be made of plastic, consisting of a chamber in which the blood was oxygenated by the injection of oxygen bubbles, topped by a debubbling chamber filled with an anti-foaming agent and a spiral reservoir [2]. New companies began to market these devices: Bentley™, Travenol™, etc. The first device of this type widely used in Europe was that of Rygg and Kyvsgaard; it was a disposable polyethylene bag containing all the components (Figure 1.3) [8]; it was widely used until the late 1970s because it was simple, effective and cheap.
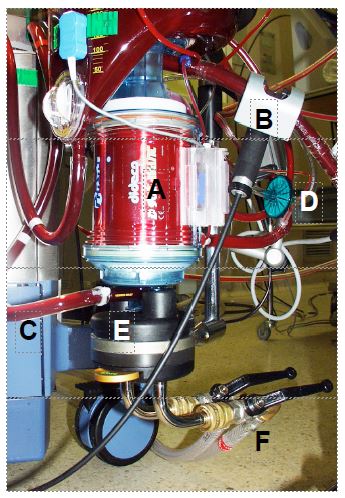
But the bubble oxygenator remained a major cause of gas embolism; moreover, once the bypass operation exceeded one hour, the direct contact of the blood with the air led to protein denaturation and complement activation, causing a massive systemic inflammatory syndrome and very often ARDS, known as pump lung. The efficiency of renal dialysis membranes led to the idea of using them to diffuse O2 without it being in direct contact with the blood. Plates of ethyl cellulose, Teflon™ and then silicone, arranged in layers or tubules, were used [6]. Although the problems of air-blood contact were solved, membrane oxygenators were slow to take off because of their complexity and price. Today, their improvements and ease of use make them the only systems used in ECC machines; they are included in a block consisting of the oxygenator, the venous reservoir and the heat exchanger (Figure 1.4).
Figure 1.4: The bypass oxygenator. It is generally coupled to the venous reservoir and the heat exchanger by the manufacturer. A: Oxygenator body containing microfibres for gas exchange. B: venous blood coming from the reservoir via the pump. C: arterial blood leaving the oxygenator. D: gas inlet (with micropore filter). E: heat exchanger. F: pipes for circulating cooling/heating water.
With the exception of the pump, all components of the ECC are now disposable. The circuit also incorporates a number of safety systems that were not present in the first models: bubble monitor, arterial and venous filters, SaO2 and SvO2 monitoring, reservoir pump control, etc. Current work is focused on improving the biocompatibility of the contact surfaces and miniaturising the whole system (see Chapter 7, Machines and Circuits).
| History of ECC |
|
A few points of reference - 1953 first successful closure of ASD in ECC - 1956 bubble oxygenator - 1967 membrane oxygenator - 2000 mini ECC |
© PG Chassot April 2007, last update September 2019
References
- DENNIS C, SPRENG DS, NELSON GE, et al. Development of a pump-oxygenator to replace the heart and lung: An apparatus applicable to human patients and an application to one case. Ann Surg 1951; 134:709-21
- DEWALL RA, WARDEN HE, GOTT VL, et al. Total body perfusion for open cardiotomy using bubble oxygenator. J Thorac Surg 1956; 32:591-603
- GIBBON JH. Artificial maintenance of circulation during experimental occlusion of the pulmonary artery. Arch Surg 1937; 34:1105-31
- GIBBON JH. Application of the mechanical heart and lung apparatus to cardiac surgery. Minn Med 1954; 36:171-85
- HOOKER DR. The perfusion of the mammalian medulla: The effect of calcium or potassium on the respiratory and cardiac centers. Am J Physiol 1915; 38:200-8
- LANDE AJ, DOS SJ, CARLSON RG, et al. A new membrane oxygenator dialyser. Surg Clin N Am 1967; 47:1461-70
- LE GALLOIS JC. Expériences sur le principe de la vie, notamment sur le siège de ce principe. Paris, D'Hautel 1812
- RYGG IH, KYVSGAARD E. A disposable polyethylene oxygenation system applied in the heart/lung machine. Acta Chir Scand 1956; 112:433-7
- STAMMERS AH. Historical aspects of cardiopulmonary bypass: From antiquity to acceptance. J Cardiothorac Vasc Anesth 1997; 11:266-74
- VON FREY M, GRUBER M. Ein respirations-apparat für isolierte organe. Arch Physiol 1885; 9:519-32