Inflammation is a defence reaction of the body against an invader. It is a complex and redundant system, with many amplifying feedback loops, but also inhibitory circuits that limit its scope. If wounded, the skin or mucous membrane barrier is broken. The body must both protect itself from toxins and prevent blood loss. This is why the inflammatory reaction is closely linked to coagulation. Normally, blood is solely in contact with the vascular endothelium, with which it maintains a constant balance. When it encounters a non-endothelialized surface such as a lesion or foreign body, it triggers a whole cascade of protective phenomena. The inflammatory process is designed to remain localised, but sometimes the response is exaggerated or the aggression is systemic, as is the case when blood comes into contact with foreign surfaces like an ECC circuit. The body's defence reaction then becomes a Systemic Inflammatory Reaction Syndrome (SIRS).
In cardiac surgery, SIRS is triggered by a range of phenomena: contact with the surfaces of the bypass circuit, contact with air in cardiotomy suctions, hypothermia, heparinisation and heparin-protamine complexes, ischaemia and reperfusion, or toxins released in the gastrointestinal tract. Approximately 20% of low-risk patients develop SIRS-related complications [19,25]: coagulopathy, interstitial fluid accumulation (cerebral oedema, gas exchange impairment), multi-organ dysfunction (neurological disorders, cardiac, renal and hepatic failure).
-
Early phase; is initiated by contact of blood with a non-endothelialized surface such as the ECC (contact pathway), and consists of two interrelated components:
- The humoral pathway, which includes 3 elements, coagulation, complement and cytokines;
- The cellular pathway, consisting of the white blood cells and the endothelium.
-
Late phase: is related to ischaemia and reperfusion injury and the release of endotoxins, mainly from the gastrointestinal tract.
Humoral pathway
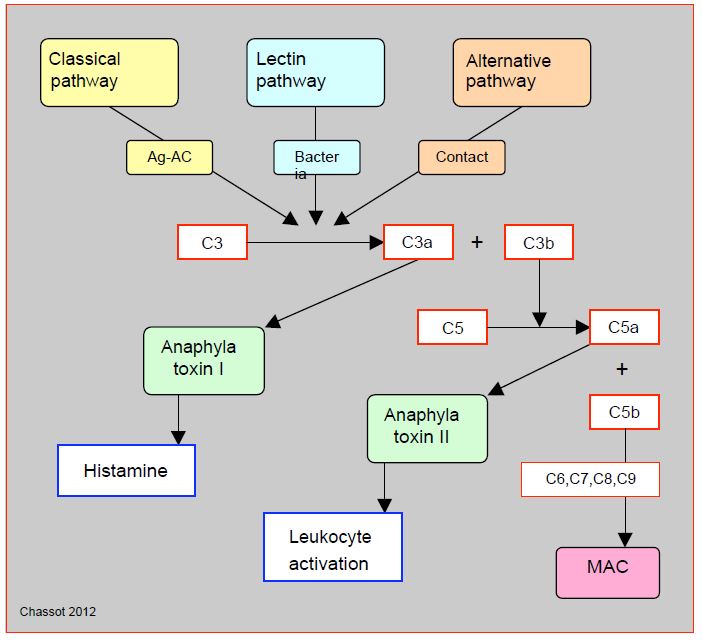
Complement is the body's oldest means of defence, as prove sea urchins. It consists of a set of 35 molecules that assist the antigen-antibody reaction or anti-bacterial defence, and which converge on specially powerful proteins which can perforate cell membranes (MAC: membrane attack complex) [49]. Closely linked to the coagulation cascade, the complement chain includess 3 distinct pathways (classical, alternative and lectin) which converge towards the cleavage of factor C3 into C3a (anaphylatoxin I, histamine release) and C3b, which in turn cleaves factor C5 into C5a (anaphylatoxin II, leukocyte activator) and C5b which is the first component of the MAC complex (Figure 7.21).
Figure 7.21: Simplified diagram of complement activation. The complement chain includes 3 pathways that are initiated by distinct triggers: the classical pathway (antigen-antibody Ag-Ac reaction), the alternative pathway (direct activation by contact) and the lectin pathway (bacterial membrane site). Through various intermediate steps, they converge on the cleavage of factor C3 into C3a (anaphylatoxin I, histamine release) and C3b, which in turn cleaves factor C5 into C5a (anaphylatoxin II, leukocyte activator) and C5b, which is the first component of the MAC (membrane attack complex), a protein complex that attacks cell membranes and is the ultimate result of the complement pathways. The many intermediate steps are not illustrated here.
The kinin-kallikrein system includes a group of serum proteins involved in the regulation of vascular tone and permeability. They circulate as inactive kininogens, and are activated by kallikreins release in injured tissue. This reaction gives rise to bradykinin which is vasodilatory (secretion of NO and prostacyclin). There is a plasma kallikrein that circulates as inactive prekallikrein and is activated by coagulation factor XIIa (Hageman factor or contact factor). As the bradykinin formed is itself an activator of Factor XII, a self-amplifying system of the link to the coagulation cascade is formed here.
Cytokines are polypeptides that ensure communication between cells and trigger specific activities depending on their targets [48].
- TNF-alpha (Tumor necrosis factor); secreted by monocytes, mast cells and T-lymphocytes in response to bacterial endotoxins, it activates the inflammatory chain. In high concentrations, it causes septic shock symptomatology with cardiodepressor, vasodilator, thrombogen and capillary permeabilising effects.
- Pro-inflammatory interleukins (IL-1, IL-6, IL-8); secreted by leukocytes, they activate the production of C-reactive protein, fibrinogen and catecholamines; they cause hyperglycaemia, leukocytosis and fever.
- Anti-inflammatory interleukins (IL-10); they provide negative feedback that prevents these chain reactions from spiralling out of control.
The coagulation cascade (see Figure 7.20) is intimately connected to the inflammatory response. Thrombin, for example, potentiates several effects: activation of polymorphonuclear cells and platelets, secretion of IL-6 and IL-8, activation of C3. Platelets in turn stimulate leukocyte adhesion to the vascular wall. Despite heparinisation, thrombin production continues and maintains a consumption coagulopathy [25].
Cellular pathway
Polymorphonuclear neutrophils responsible for phagocytosis of pathogens contain granules that can release proteolytic enzymes, oxygen radicals and inflammatory mediators. When vascular injury occurs, they first roll onto the surface of the endothelium through the attachment of molecules called selectins, then adhere to the injured endothelium through the anchoring of molecules called integrins (adhesion molecules). Thirdly, they migrate into the interstitial space by chemotaxis to release their bactericidal substances [45].
Basophils, activated by the complement pathway, mainly secrete histamine, which increases capillary permeability and eases the transfer of formed elements; it also causes vasodilation and bronchoconstriction. Mast cells look alike basophils but are confined to the perivascular space of organs and do not circulate; when stimulated by complement or thrombin, they secrete various inflammatory mediators including many interleukins. Finally, monocytes are macrophages that release a series of inflammatory factors (interleukins, TNF-alpha) and phagocyte foreign elements [48].
Endothelial cells are the natural regulators of coagulation and inflammation. They respond to the presence of thrombin, factor C5a, cytokines and interleukins by secreting selectins and integrins that immobilise macrophages. They manage vascular tone through the release of vasodilator substances such as NO, histamine and bradykinin or vasoconstrictor substances such as endothelin-1 or noradrenaline; in addition, NO inhibits platelet function.
In addition to leukocytes, ECC also activates thrombocytes, which will secrete coagulation factors, cytokines and wall adhesion molecules [25].
Late phase
The late phase has two components.
- Ischaemia and reperfusion injury. After being deprived of O2 during ischaemia, the cell receives a large amount of O during reperfusion, which triggers a cascade of pathological events that are more severe than the damage caused by ischaemia through the massive formation of peroxides (free radicals, ROS reactive oxygen species) that contain an odd number of electrons in their outer orbital: peroxide (O2- ), H2 O2 , hydroxyl (•OH) (see Chapter 24, Mechanisms). Peroxides released during leukocyte activation or during reperfusion after ischaemia trigger a major inflammatory reaction. Normally antagonized by natural antioxidants (superoxide dismutase, catalase, glutathione, vitamin E), they spill over into the extracellular fluid when produced en masse and attack membrane phospholipids, DNA and proteins [12,15].
- Endotoxins massively stimulate the production of complement, TNF-alpha and interleukins. Endotoxin release from Gram-negative bacteria present in the gastrointestinal tract is related to the decrease in splanchnic flow in bypass surgery and hypothermia; flow in the gastric mucosa, for example, is decreased by up to 60% through low flow and vasoconstriction [38].
The global inflammatory response leads to multiple organ dysfunctions: ARDS, renal or liver failure.
Special case: the ECC
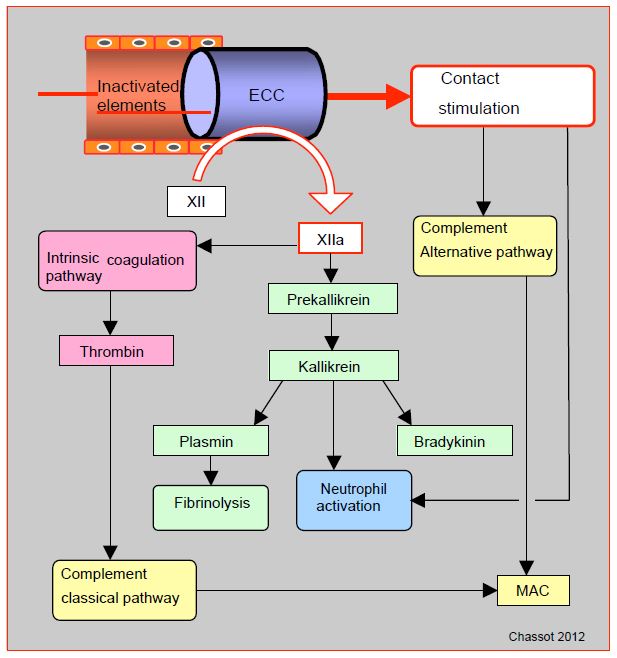
ECC is the most emblematic case of contact stimulation. In the presence of negatively charged surfaces such as glass, metals or plastics, Factor XII cleaves to F XIIa (activated). This converts prekallikrein to kallikrein, kininogens to bradykinin and factor XI to F XIa; the latter process leads to the formation of thrombin via the intrinsic coagulation pathway. F XIIa also promotes the conversion of plasminogen to plasmin, resulting in fibrinolysis. Contact activates complement directly through the alternative pathway, and indirectly through F XIIa (classical pathway). Other phenomena in the ECC contribute to complement activation, such as the release of endotoxins and the formation of heparin-protamine complexes. The cellular pathway is also stimulated by contact, either via factor XIIa and kallikrein or directly by neutrophil activation. However, the extracorporeal circuit has no endothelium to limit these different reactions, which can therefore become excessive and dissiminated throughout the body (Figure 7.22).
The duration of bypass surgery, the depth of hypothermia, the degree of haemodilution and genetic factors have all been mentioned as aggravating factors, but they appear to have only a secondary role in the genesis of SIRS [22]. Mechanical damage due to pump, oxygenator and filters, contact of the blood with foreign surfaces (circuits) and with air (aspirations, venous reservoir) are the main triggers. More than 50% of neutrophils are sequestered in the lungs during rewarming; their degranulation contributes to lung cell damage. The SIRS is triggered in the first minutes of the bypass surgery and disappears by 4ème - 5ème postoperative day, followed by a period of relative immunosuppression [41]. The peak of inflammatory markers occurs at around the 5th hour after bypass surgery [12].
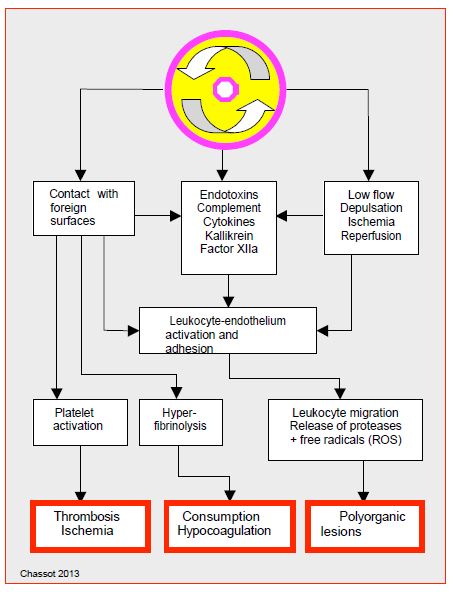
During ECC, inflammatory stimulation thus leads to an unstable state characterised by a series of phenomena (Figure 7.23).
- Elevation of all inflammatory markers (TNF-alpha, interleukins, cytokines, C-reactive protein);
- Activation of complement and leukocytes;
- Production of thrombin and stimulation of the coagulation cascade;
- Production of plasmin and stimulation of fibrinolysis (elevation of D-dimer);
- Activation and consumption of platelets;
- Release of endotoxins and TNF-alpha;
- Release of free radicals and oxidants;
- Release of bradykinin, histamine and anaphylatoxins: increased capillary permeability, systemic vasodilation, pulmonary vasoconstriction;
- Alterations in cardiac, pulmonary, renal and cerebral function; the incidence of atrial fibrillation is proportional to the elevation of inflammatory markers [14].
Figure 7.22: Activation via the contact pathway. In a vessel layered with intact endothelium, the various factors circulate in inactive form. But in the presence of negatively charged surfaces such as glass, metals or plastics (ECC), Factor XII cleaves to F XIIa (activated). This converts prekallikrein to kallikrein, kininogens to bradykinin and factor XI to F XIa; the latter process leads to the formation of thrombin via the intrinsic coagulation pathway. F XIIa also promotes the conversion of plasminogen to plasmin, causing fibrinolysis. Contact activates complement directly through the alternative pathway, and indirectly through F XIIa (classical pathway). MAC (membrane attack complex): a protein complex that attacks cell membranes, the ultimate result of the complement pathways.
The question of how much the genesis of SIRS is due to bypass surgery versus cardiac surgery itself can be approached by looking at beating heart surgery. The latter is associated with a reduction, but not an elimination, of postoperative levels of inflammatory response markers such as C3a, C5a, TNF-alpha or interleukin-6 and -8 [8,9,18]. However, the significance of these variations is uncertain, as IL-8 and C3a are related to direct tissue trauma and IL-10 has anti-inflammatory properties [34]. The effects of ECC depend largely on the balance between the release of pro- and anti-inflammatory mediators [26]. Some populations show a dominant pro-inflammatory response, such as the elderly or those with left ventricular dysfunction [44]. They may particularly benefit from beating heart surgery. In low-risk groups, SIRS-related complication rates are identical between operations with and without bypass surgery [36,40]. The bypass circuit is therefore a major triggering factor, but not the only one responsible for the inflammatory response [52].
Figure 7.23: Schematic representation of the mechanisms involved in the genesis of systemic inflammatory syndrome [22,47].
Technical improvements
Apart from beating heart procedures, various technical means aim at reducing the inflammatory syndrome triggered by bypass surgery, but none of them can eliminate it. The clinical significance of these improvements is modest at most [30,55].
- Suction restriction; sucked blood contains air, cellular debris and activators of coagulation (TNF, thrombin, plasmin) or inflammation (interleukins, C3a, C5a). Suctions are the main source of embolism, haemolysis, thrombocytopenia, coagulopathy and SIRS stimulation [47]. Disruption of the coagulatory system is markedly reduced when sucked blood is not recycled or is filtered by a CellSaver™ system, alas these manoeuvres remove platelets, proteins and coagulation factors [53].
- Restriction of circuit size; miniaturisation of circuits and elimination of the cardiotomy reservoir (MECC: mini-extracorporeal circuit) minimises blood contact with foreign surfaces and eliminates contact with air, thus reducing the release of coagulation activators and inflammatory triggers (see Mini-ECC) [3,58].
- Biocompatibility of circuits; preheparinised circuits and circuits impregnated with polymers such as poly-2-methoxy-ethyl-acrylate inhibit the complement cascade and leukocyte activation, reduce platelet adhesiveness and coagulation factor adsorption [20]. Although they reduce pulmonary, renal and neurological complications, their clinical impact is not significant [32,50].
- Haemofiltration; reduces excess fluid specific to bypass surgery and removes water-soluble inflammatory triggers. It reduces postoperative interstitial oedema, transfusion requirements, systemic inflammatory syndrome and multi-organ failure [5,7,29,42].
- Leukocyte filters; reducing leukocyte levels is beneficial especially for gas exchange, since the lungs are the main site of leukocyte sequestration during bypass surgery. Unfortunately, these filters do not prevent postoperative respiratory failure [2].
- Normothermia; maintaining temperature ≥ 34°C avoids coagulation alterations and inflammatory flare-up triggered by rewarming, but the ideal temperature for bypass surgery has not yet been defined [37,43].
These improvements are likely to have an impact on postoperative complications in high-risk or complex procedures, but have little influence on standard procedures in low- to moderate-risk patients. Given the increased cost, these new technologies do not currently have a favourable cost/benefit ratio for routine use. Normothermia by itself costs nothing,
Steroids
Corticosteroids maintain the integrity of cell membranes, particularly in the heart and lungs, inhibit leukocyte adhesion and reduce complement and cytokine production [23,54,55]. Despite a clear attenuation of the inflammatory response and a clear decrease in inflammatory markers after ECC, they only prevent multi-organ dysfunction in hypocortic patients, and their clinical impact remains unclear. Various reviews and meta-analyses have summarised the results obtained so far with steroids [1,6,35,56].
- The reduction in the incidence of AF is significant, but this effect is clear in studies of small scale populations [11].
- ICU stay and hospital stay are reduced.
- Intraoperative haemodynamic stability is improved, the use of vasopressor is reduced.
- The reduction of bleeding is marginal.
- The duration of mechanical ventilation is somewhat shortened; some studies have shown an attenuation of respiratory complications, but other publications show a worsening of postoperative gas exchange and a delay in extubation.
- Waking up is improved: the rate of nausea, vomiting and shivering is reduced.
- The rate of wound infection is the same, and the rate of general infections is marginely modified.
- The rate of hyperglycaemic episodes is increased 1.5 times in one meta-analysis and the rate of GI bleeding is doubled in another.
- Mortality is not changed.
Although the protocols of the different studies vary greatly, it can be concluded that prophylactic steroid administration may reduce postoperative morbidity, provided that it does not itself cause increased infection and bleeding [1]. As the traditional dosages are quite high (methylprednisolone 30 mg/kg, dexamethasone 1 mg/kg), it is possible that lower doses may have a better benefit/complication ratio while maintaining satisfactory activity in reducing the inflammatory response [35].
The question of whether routine steroid prophylaxis is advisable has been answered rather negatively by two randomised controlled trials. The first is a Dutch study (DECS, DExamethasone for Cardiac Surgery) of 4,494 patients randomised to dexamethasone 1 mg/kg or placebo, administered at induction of anaesthesia [10]. Mortality and major complications (MI, stroke, renal failure) were marginally lower in the steroid group (OR 0.83), while other morbidities were significantly reduced: infections (OR 0.64), delirium (OR 0.79), respiratory failure (OR 0.69). High-risk patients (EuroSCORE > 5) benefit the most (OR 0.77). Although these results are not overwhelming, this trial clearly demonstrated the safety of the treatment, which did not trigger any worsening of the bleeding or infectious risk. The second trial was a multicentre study (SIRS, Steroids In caRdiac Surgery) of 7,507 patients assigned to receive 500 mg methylprednisolone or placebo [57]. Mortality was not significantly reduced (OR 0.87), the rate of major complications was unchanged (OR 1.03) and the rate of infection was unchanged, as was the incidence of delirium. These two studies, which are methodologically indisputable, do not therefore encourage the use of steroids as a routine to reduce the rate of complications related to the inflammatory reaction of the ECC.
Other substances
Antifibrinolytics bind to the lysine of plasminogen and block the activation of plasmin, thus fibrinolysis. In clinical practice, they reduce blood loss by 30% overall. Three substances are used for this purpose (see Antifibrinolytics).
- Aprotinin is a non-specific inhibitor of many proteases that directly blocks plasmin; it inhibits complement, TNF and kallikrein production [26]. It was withdrawn from the market in November 2007 due to excess renal failure, cardiac complications and mortality, although it was more effective than the other two substances in reducing bleeding risk and transfusion rates [13,31].
- Tranexamic acid does not trigger allergic reactions or renal dysfunction, but high doses increase the risk of postoperative seizures [24,33]. Although it effectively reduces the risk of bleeding, it has little anti-inflammatory effect.
- e -aminocaproic acid is slightly less effective [24].
A wide range of pharmacological substances are potentially useful in alleviating the inflammatory syndrome, although it cannot be demonstrated that their clinical impact is significant.
- Complement inhibition: pexelizumab and TP10, which inhibit the complement chain at C5a, reduce morbidity and mortality after cardiac surgery in selected groups of patients, but have not yet shown significant clinical effects [49].
- Antioxidants: N-acetylcysteine may improve postoperative lung function [16]; ascorbic acid, alpha-tocoferol and allopurinol detoxify free radicals, but clinical studies have not found significant benefit [17].
- Pentoxifyline (theophylline analogue): inhibits mediators and the complement cascade, and appears to improve postoperative ventilation [4].
- Statins have an anti-inflammatory effect and reduce mortality after coronary artery bypass surgery [59].
- Methylene blue is an anti-NO and therefore has an anti-inflammatory effect, but its use is limited to situations of refractory hypotension [27].
- Insulin; hyperglycaemia is accompanied by hypersecretion of cytokines, and diabetics are known to produce excessive superoxide from their mitochondria. Therefore, maintaining normoglycaemia attenuates the inflammatory response, but the latter induces insulin resistance which may explain the challenge of managing intraoperative blood glucose [46].
- Various agents have some anti-inflammatory effect: milrinone, nitroprusside, morphine, heparin, ACE inhibitors [55].
Anaesthetic aspects
The anaesthetist can influence the inflammatory response in several ways [21].
- Ventilation: lowering the tidal volume to 4-8 mL/kg instead of 10-12 mL/kg, limiting the Pplateau to < 30 cm H2 O, lowering the FiO2 to 0.3-0.6, and performing recruitment manoeuvres every 30 minutes limit mechanical and cellular damage, and lower the levels of cytokines released from the lungs [28].
- Haemodynamic stability: tissue hypoperfusion (hypotension and low flow) activates the release of cytokines and endotoxins, particularly in the gastrointestinal tract.
- Preconditioning: Halogens have the property of reducing the impact of ischaemic injury on the myocardial cell, at least momentarily (see Chapter 5 Conditioning) [39]. Remote preconditioning with short periods of muscular ischaemia of a limb has a protective effect on the myocardium and clinical survival [51].
| Systemic inflammatory reaction |
|
The inflammatory response is a rapid, complex and redundant system that aims at protecting the body against aggressors. It includes a humoral pathway (complement, cytokines, kinins), a cellular pathway (leukocytes, endothelium) and a late phase (ischemia-reperfusion injury, release of endotoxins). It is highly intertwined with the coagulation cascade.
Direct contact of blood with the bypass circuit and air induces a massive systemic inflammatory response (SIRS) triggered by activation of Factor XII (Hageman), complement and leukocytes. It is characterised by:
- Elevation of all inflammatory markers
- Activation of complement and leukocytes
- Thrombin production and stimulation of the coagulation cascade
- Plasmin production and stimulation of fibrinolysis
- Platelet activation and consumption
- Release of endotoxins, TNF-alpha and interleukins
- Release of free radicals and oxidants
- Release of bradykinin, histamine and anaphylatoxins
- Increase in capillary permeability, decrease in SAR, increase in PAR
- Alterations in heart, lung, kidney and brain function
There are many treatment options, but they only have a clinical impact in high-risk cases:
- Restriction of contact with foreign surfaces (mini-circuits, biocompatible materials)
- Restriction of air contact (limitation of suction, no venous reservoir)
- Hemofiltration
- Antifibrinolytics
- Corticosteroids
- Antioxidants, complement inhibitors
- Operation without ECC
|
© CHASSOT PG, GRONCHI F, April 2008, last update December 2019
References
- AUGOUSTIDES JGT. The inflammatory response to cardiac surgery with cardiopulmonary bypass: shoud steroid prophylaxis be routine? J Cardiothorac Vasc Anesth 2012; 26 :952-8
- ASIMAKOPOULOS G. The inflammatory response to CPB: the role of leukocyte filtration. Perfusion 2002; 17:7-10
- BIANCARI F, RIMPILAINEN R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009; 95:964-9
- BOLDT J, BROSCH C, LEHMANN A, et al. Prophylactic use of pentoxifylline on inflammation in elderly cardiac surgery patients. Ann Thorac Surg 2001; 71:1524-9
- BOODHWANI M, WILLIAMS K, BABAEV A, et al. Ultrafiltration reduces blood transfusion following cardiac surgery: a meta-analysis. Eur J Cardiothorac Surg 2006; 30:892-7
- CAPPABIANCA G, ROTUNNO C, DE LUCA TUPPUTI L, et al. Protective effects of steroids in cardiac surgery: A meta-analysis of randomized double-blind trials. J Cardiothorac Vasc Anesth 2011; 25:156-65
- CHEW MS, BRIX-CHRISTENSEN V, RAVN HB, et al. Effect of modified ultrafiltration on the inflammatory response in paediatric open-heart surgery: a prospective, randomized study. Perfusion 2002; 17:327-33
- Czerny M, Baumer H, Kilo J, et al. Inflammatory response and myocardial injury following coronary artery bypass grafting with or without cardiopulmonary bypass. Eur J Cardiothorac Surg 2000; 17: 737-42
- Diegeler A, Doll N, Rauch T, et al. Humoral immune response during coronary artery bypass grafting: A comparison of limited approach, "off-pump" technique, and conventional cardiopulmonary bypass. Circulation 2000; 102: III95-100
- DIELMAN JM, NIERICH AP, ROSSEEL PM, et al. Intraoperative high-dose dexamethasone in cardiac surgery:a randomized controlled trial. JAMA 2012; 308:1761-7
- DVIRNIK N, BELLEY-COTE EP, HANIF H, et al. Steroids in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth 2018; 120:657-67
- ELAHI MM, YII M, MATATA BM. Significance of oxidants and inflammatory mediators in blood of patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2008; 22:455-67
- FERGUSSON DA, HEBERT PC, MAZER CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med 2008; 358:2319-31
- FONTES ML, MATHEW JP, RINDER HM, et al. Atrial fibrillation after cardiopulmonary bypass is associated with monocyte activation. Anesth Analg 2005; 101:17-23
- FRÄSSDOR J, DE HERT S, SCHLACK W. Anaesthesia and myocardial ischaemia/reperfusion injury. Br J Anaesth 2009; 103:89-98
- FRUMENTO RJ, LEE D, OZ MC, et al. N-acetylcysteine and total antioxidant status in high-risk cardiac surgical patients. Anesth Analg 2002; 93:SCA16
- GIRARD C. ECC and inflammation. In: JANVIER G, LEHOT JJ (ed). Circulation extracorporelle: principes et pratique, 2nd edition. Paris, Arnette Groupe Liaison SA, 2004, pp 147-55
- GORMLEY SMC, McBRIDE WT, ARMSTRONG MA, et al. Plasma and urinary cytokine homeostasis and renal function during cardiac surgery without cardiopulmonary bypass. Cytokine 2002; 17:61-5
- GROVER FL. The Society of thoracic Surgeons national Database. Current status and future directions. Ann Thorac Surg 1999; 68:367-73
- GUNAYDIN S, FARSAK B, KOKAKULAK M, et al. Clinical performance and biocompatibility of poly(2-methoxyethylacrylate)-coated extracorporeal circuits. Ann Thorac Surg 2002; 74:819-24
- HALL R. Identification of inflammatory mediators and their modulation by strategies for the management of the Systemic Inflammatory Response during cardiac surgery. J Cardiothorac Vasc Anesth 2013; 27: 983-1033
- HENNEIN HA. Inflammation after cardiopulmonary bypass: therapy for the postpump syndrome. Semin Cardiothorac Vasc Anesth 2001; 5:236-55
- KILGER E, WEISS F, BRIEGEL J, et al. Stress doses of hydrocortisone reduce severe systemic inflammatory response syndrome and improve early outcome in a risk group of patients after cardiac surgery. Crit Care Med 2003; 31:1068-74
- KOSTER A, SCHIRMER U. Re-evaluation of the role of antifibrinolytic therapy with lysine analogs during cardiac surgery in the post aprotinin era. Curr Opin Anaesthesiol 2011; 24:92-7
- KRAFT F, SCHMIDT C, VAN AKEN H, ZARBOCK A. Inflammatory response and extracorporeal circulation. Best Pract Res Clin Anaesthesiol 2015; 29:113-23
- Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 2002; 97: 215-52
- LAVIGNE D, Vasopressin and methylene blue: alternate therapies in vasodilatory shock. Semin Cardiothorac Vasc Anesth 2010; 14:186-9
- LELLOUCHE F, DELORME M, BUSSIÈRES J, OUATTARA A. Perioperative ventilatory strategies in cardiac surgery. Best Pract Res Clin Anaesthesiol 2015; 29:381-95
- LUCIANI GB, MENON T, VECCHI B, et al. Modified ultrafiltration reduces morbidity after adult cardiac operations: a prospective randomized clinical study. Circulation 2001: 104:1253-9
- MAHARAJ C, LAFFEY JG. New strategies to control the inflammatory response in cardiac surgery. Curr Opin Anesthesiol 2004; 17:35-48
- MANGANO DT, TUDOR I, DIETZEL C, et al. The risk associated with aprotinin in cardiac surgery. N Engl J Med 2006; 354:353-65
- MANGOUSH O, PURKAYASTHA S, HAJ-YAHIA S, et al. Heparin-bonded circuits versus nonheparin-bonded circuits: an evaluation of their effect on clinical outcomes. Eur J Cardiothorac Surg 2007; 31:1058-69
- 33MARTIN K, WIESNER G, BREUER T, et al. The risks of aprotinin and tranexamic acid in cardiac surgery: A one-year follow-up of 1188 consecutive patients. Anesth Analg 2008; 107:1783-90
- Menasche P. The systemic factor: the comparative roles of cardiopulmonary bypass and off-pump surgery in the genesis of patient injury during and following cardiac surgery. Ann Thorac Surg 2001; 72: S2260-5; discussion S5-6
- MURPHY GS, WHITLOCK RP, GUTSCHE JT, et al. Steroids for adult cardiac surgery with cardiopulmonary bypass: update on dose and key randomized trials. J Cardiothorac Vasc Anesth 2013; 27:1053-9
- Nathoe HM, Van Dijk D, Jansen EWL, et al. A comparison of on-pump and off-pump coronary bypass surgery in low-risk patients. N Engl J Med 2003; 348:394-402
- OHATA T, SAWA Y, KADOBA K, et al. Effect of cardiopulmonary bypass under tepid temperature on inflammatory reactions. Ann Thorac Surg 1997; 64:124-8
- OPAL SM. The host response to endotoxin, antipolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol 2007; 297:365-77
- PAGEL PS. Myocardial protection by volatile anesthetics in patients undergoing cardiac surgery: a critical review of the laboratory and clinical evidence. J Cardiothorac Vasc Anesth 2013; 27:972-82
- Puskas JD, Williams WH, Duke PG, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: A prospective randomized comparison of two-hundred unselected patients undergoing off-pump versus conventional coronary artery bypass surgery. J Thorac Cardiovasc Surg 2003; 125:797-808
- RINDER C. Cellular inflammatory response and clinical outcome in cardiac surgery. Curr Opin Anaesthesiol 2006; 19:65-8
- SEARLES B, DARLING E. Ultrafiltration in cardiac surhery. In: MONGERO LB, BECK JR (eds). On bypass. Advanced perfusion techniques. Totowa (NJ, USA): Humana Press 2010, 193-210
- SHANN KG, LIKOSKY DS, MURKIN JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: A focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg 2006; 132:283-90
- Sharony R, Bizekis CS, Kanchuger M, et al. Off-pump coronary artery bypass grafting reduces mortality and stroke in patients with atheromatous aortas: A case control study. Circulation 2003; 108 (suppl II):15-20
- SHERWOOD ER, TOLIVER-KINSKY T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol 2004; 18:385-405
- SMITH JW, ROMIJN JA. Acute insulin resistance in myocardial ischemia: causes and consequences. Semin Cardiothorac Vasc Anesth 2006; 10:215-9
- SNIECINSKI RM, CHANDLER WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113:1319-33
- SNIECINSKI RM, LEVY JH. The inflammatory response to cardiopulmonary bypass. In: MONGERO LB, BECK JR (eds). On bypass. Advanced perfusion techniques. Totowa (NJ, USA): Humana Press 2010, 125-140
- 49STAHL GL, SHERMAN SK, SMITH PK, LEVY JH. Complement activation and cardiac surgery: a novel target for improving outcomes. Anesth Analg 2012; 115:759-71
- 50 SVENMARKER S, HAGGMARK S, JANSSON E, et al. Use of heprin-bonded circuits in cardiopulmonary bypass improves clinical outcome. Scand Cardiovasc J 2002; 36:241-6
- THIELMANN M, KOTTENBERG E, KLEINBONGARD P, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet 2013; 382:597-604
- UNTCH BR, JESKE WP, SCHWARTZ J, et al. Inflammatory and hemostatic activation in patients undergoing off-pump coronary artery bypass grafting. Clin Appl Thromb Hemost 2008; 14-141-8
- WANG G, BAINBRIDGE D, MARTIN J, et al. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg 2009; 109:320-30
- WARREN OJ, SMITH AJ, ALEXIOU C, et al. The inflammatory response to cardiopulmonary bypass: Part 1 - Mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009; 23: 223-31
- WARREN OJ, WATRET AL, DE WIT KL, et al. The inflammatory response to cardiopulmonary bypass: Part 2 - Anti-inflammatory therapeutic strategies. J Cardiothorac Vasc Anesth 2009; 23: 384-93
- WHITLOCK RP, CHAN S, DEVEREAUX PI, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: A meta-analysis of randomized trials. Eur Heart J 2008; 29: 2592-600
- WHITLOCK RP, DEVEREAUX PJ, TEOH KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 26:1243-53
- ZANGRILL A, GAROZZO FA, BIONDI-ZOCCAI G, et al. Miniaturized cardiopulmonary bypass improves short-term outcome in cardiac surgery: a meta-analysis of randomized controlled trials. J Thorac Cardiovasc Surg 2010; 139:1162-9
- ZHENG Z, JAYARAM R, JIANG L, et al. Perioperative rosuvastatin in cardiac surgery. N Engl J Med 2016; 374:1744-53