Inflammation is a defence reaction of the body against an invader. It is a complex and redundant system, with many amplifying feedback circuits, but also inhibitory ones that limit its scope. In case of a wound, skin or mucous membrane barrier is broken. The body must both protect itself from toxins and prevent blood loss. This is why the inflammatory reaction is closely linked to coagulation. Normally, blood is in sole contact with the vascular endothelium, with which it maintains a constant balance. When it encounters a non-endothelialized surface such as a lesion or foreign body, it triggers a whole cascade of protective phenomena. The inflammatory process is designed to remain localised, but sometimes the response is amplified or the aggression is systemic, when for example blood comes into contact with ECC surfaces. The body's defence reaction then becomes a Systemic Inflammatory Reaction Syndrome (SIRS). The clinical picture is typical: fever, tachycardia, hypotension, leukocytosis, fluid accumulation and polyorgan failure [4].
In cardiac surgery, SIRS is triggered by a range of phenomena: contact with bypass circuit surfaces, contact with air in cardiotomy suctions, hypothermia, heparinisation and heparin-protamine complexes, ischaemia and reperfusion injury, or toxins released in the gastrointestinal tract. Approximately 20% of low-risk patients develop SIRS-related complications [3]: coagulopathy, interstitial fluid accumulation, cerebral oedema, pulmonary oedema (impaired gas exchange), multi-organ dysfunction (neurological disorders, cardiac, renal and hepatic failure).
The inflammatory response consists of two distinct phases with separate triggers [7,9].
- Early phase; is initiated by contact of blood with an endothelial lesion or a non-endothelialized surface such as the ECC circuit (contact pathway), and consists of two interrelated components:
- The humoral pathway, which includes 3 elements, coagulation, complement and cytokines;
- The cellular pathway, involving white blood cells and endothelium.
- Late phase: is related to ischaemia and reperfusion injury and the release of endotoxins, mainly from the gastrointestinal tract.
Humoral pathway
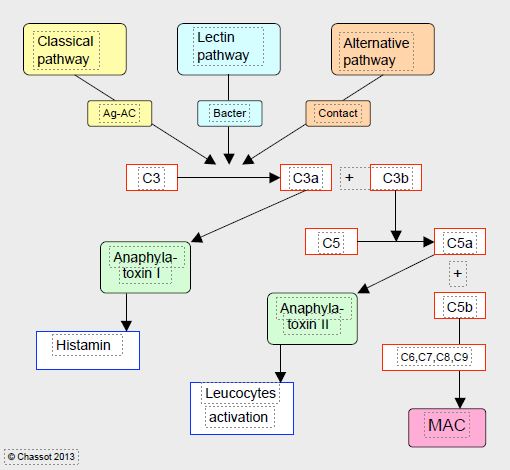
The complement pathway is the body's oldest mean of defence, it was already found in sea urchins. It consists of a set of 35 molecules that assist the antigen-antibody reaction or anti-bacterial defence, and converge on incredibly amazing proteins able to perforate cell membranes (MAC: membrane attack complex) (Figure 8.8) [8].
Figure 8.8: Simplified diagram of complement activation. The complement chain comprises 3 pathways that are initiated by distinct triggers: the classical pathway (antigen-antibody Ag-Ac reaction), the alternative pathway (direct activation by contact) and the lectin pathway (bacterial membrane site). Through various intermediate steps, they converge on the cleavage of factor C3 into C3a (anaphylatoxin I, histamine release) and C3b, which in turn cleaves factor C5 into C5a (anaphylatoxin II, leukocyte activator) and C5b, which is the first component of the MAC (membrane attack complex), a protein complex that attacks cell membranes and is the ultimate result of the complement pathways. The many intermediate steps are not described in this diagram.
Closely interwoven with coagulation cascade, complement chain involves 3 distinct pathways (classical, alternative and lectin) that converge to cleave factor C3 into C3a (anaphylatoxin I, histamine release) and C3b, which in turn cleaves factor C5 into C5a (anaphylatoxin II, leukocyte activator) and C5b which is the first component of MAC complex.
The kinin-kallikrein system is part of a group of serum proteins involved in the regulation of vascular tone and permeability. They circulate as inactive kininogens, which are activated by kallikreins released in injured tissue. This reaction gives rise to bradykinin which is vasodilatory (secretion of NO and prostacyclin). A plasma kallikrein circulates as inactive prekallikrein and is activated by coagulation factor XIIa (intrinsic pathway). As the bradykinin formed is itself an activator of Factor XII, a self-amplifying system of the link with coagulation cascade is formed here.
Cytokines are polypeptides that allow communication between cells and trigger specific activities depending on their targets [7].
- TNF-alpha (Tumor necrosis factor); secreted by monocytes, mast cells and T-cells in response to bacterial endotoxins, it activates the inflammatory chain. In high concentrations, it causes septic shock symptomatology with cardiodepression, vasodilation, thrombogen and capillary permeabilising effects.
- Pro-inflammatory interleukins (IL-1, IL-6, IL-8); secreted by leukocytes, they activate the production of C-reactive protein, fibrinogen and catecholamines; they cause hyperglycaemia, leukocytosis and fever.
- Anti-inflammatory interleukins (IL-10); they provide negative feedback that prevents these chain reactions from spining out of control.
The coagulation cascade (see Figure 8.1) is intimately connected to the inflammatory response. Thrombin, for example, potentiates several effects: activation of polymorphonuclear cells and platelets, secretion of IL-6 and IL-8, activation of C3. Platelets, in turn, stimulate leukocyte adhesion to the vascular wall.
Cellular pathway
Polymorphonuclear neutrophils responsible for phagocytosis of pathogens contain granules that can release proteolytic enzymes, oxygen radicals and inflammatory mediators. When vascular injury occurs, they first roll onto the surface of the endothelium through the attachment of molecules called selectins, and then adhere to the injured endothelium through anchoring of molecules called integrins (adhesion molecules). Thirdly, they migrate into the interstitial space by chemotaxis to release their bactericidal substances [6].
Basophils, activated by complement pathway, mainly secrete histamine, which increases capillary permeability and facilitates passage of blood elements; it also causes vasodilation and bronchoconstriction. Mast cells resemble basophils but are confined to the perivascular space of organs and do not circulate; when stimulated by complement or thrombin, they secrete various inflammatory mediators including many interleukins. Finally, monocytes are macrophages that release a series of inflammatory factors (interleukins, TNF-alpha) and phagocyte foreign elements [7].
Endothelial cells are the natural regulators of coagulation and inflammation. They respond to the presence of thrombin, factor C5a, cytokines and interleukins by secreting selectins and integrins that immobilise leukocytes. They manage vascular tone through the release of vasodilator substances such as NO, histamine and bradykinin or vasoconstrictor substances such as endothelin-1 or noradrenaline, and NO inhibits platelet function.
Late phase
Late phase consists of two main components.
- Ischaemia and reperfusion injury. After being deprived of O2 during ischaemia, cells receive a massive amount of O2 during reperfusion, which triggers a cascade of pathological events more severe than the damage of ischaemia, notably through the massive formation of free radicals (ROS reactive oxygen species) that contain an odd number of electrons in their outer orbit: peroxide (O2- ), H2 O2 , hydroxyl (• OH) (see Chapter 24 Mechanisms). ROS released into cytoplasm by mitochondria are normally reduced by natural antioxidants (superoxide dismutase, catalase, glutathione, vitamin E), but these are overwhelmed by an abundance of peroxides formed during reperfusion. ROS can then attack membrane phospholipids, DNA and various proteins [1,2,4]. Mitochondria intoxicated by excess O2 also release cytokines that, together with peroxides, activate dangerous cytoplasmic enzymes (caspases) involved in apoptosis that can lyse cell components and lead to necrosis.
- Endotoxins massively stimulate the production of complement, TNF-alpha and interleukins. Endotoxin release from Gram-negative bacteria invading the gastrointestinal tract is related to splanchnic flow decrease during ECC and hypothermia; flow in the gastric mucosa, for example, is decreased by up to 60% by low flow (< 2 L/min/m2 ) and by vasoconstriction during bypass [5].
| Inflammation and complement |
| The inflammatory response is a rapid, complex and redundant system that aims at protecting the body against aggressors. It is highly intertwined with coagulation cascade. If response is exaggerated or the aggression is systemic, inflammation results in a systemic inflammatory syndrome (SIRS). It has three aspects.
- Humoral pathway: complement cascade producing molecules capable of lysin foreign cells, cytokines (specific triggers), kinins (tonus regulators) and vascular permeability)
- Cellular pathway: neutrophils (phagocytosis), basophils (histamine release endothelium (regulatory factors)
- Late phase: ischaemia-reperfusion injury, release of digestive endotoxins
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- ELAHI MM, YII M, MATATA BM. Significance of oxidants and inflammatory mediators in blood of patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 2008; 22:455-67
- FRÄSSDORF J, DE HERT S, SCHLACK W. Anaesthesia and myocardial ischaemia/reperfusion injury. Br J Anaesth 2009; 103:89-98
- GROVER FL. The Society of thoracic Surgeons national Database. Current status and future directions. Ann Thorac Surg 1999; 68:367-73
- HALL R. Identification of inflammatory mediators and their modulation by strategies for the management of the Systemic Inflammatory Response during cardiac surgery. J Cardiothorac Vasc Anesth 2013; 27: 983-1033
- OPAL SM. The host response to endotoxin, antipolysaccharide strategies, and the management of severe sepsis. Int J Med Microbiol 2007; 297:365-77
- SHERWOOD ER, TOLIVER-KINSKY T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol 2004; 18:385-405
- SNIECINSKI RM, LEVY JH. The inflammatory response to cardiopulmonary bypass. In: MONGERO LB, BECK JR (eds). On bypass. Advanced perfusion techniques. Totowa (NJ, USA): Humana Press 2010, 125-140
- STAHL GL, SHERMAN SK, SMITH PK, LEVY JH. Complement activation and cardiac surgery: a novel target for improving outcomes. Anesth Analg 2012; 115:759-71
- WARREN OJ, SMITH AJ, ALEXIOU C, et al. The inflammatory response to cardiopulmonary bypass: Part 1 - Mechanisms of pathogenesis. J Cardiothorac Vasc Anesth 2009; 23: 223-31