Haemodynamic management depends on the degree of right-sided dysfunction. The aim is to reduce RV afterload to facilitate anterograde ejection and reduce the regurgitant fraction. In secondary TI, management is guided by the treatment of the left-sided lesions, most commonly mitral disease. The following recommendations apply only to isolated severe TI.
- Preload: increasing preload is the physiological way for the hypertrophied RV to improve its systolic performance by using the Starling phenomenon; but if the failure has already led to ventricular dilatation and upstream stasis with tricuspid insufficiency, preload must be reduced (nitrate derivatives, diuretics, counter-Trendelenburg position). Filling must be adjusted according to right hemodynamics: PAP and cardiac output via pulmonary catheter, RV systolic function on TEE, variations in TI. CVP is an unreliable criterion for blood volume and tricuspid regurgitation because the RA is very compliant and accepts large volumes with small variations in pressure.
- Systemic Resistances: Because of the risk of RV ischaemia and leftward tilt of the interventricular septum in systemic hypotension, SAR must be maintained by infusion of norepinephrine to ensure coronary perfusion and septal balance. As the pulmonary vascular bed is very poor in alpha receptors, there is no risk of a significant increase in pulmonary resistance.
- Pulmonary Resistance: The size of the TI is directly proportional to the size of the PAR. In addition, the RV is less able to withstand an increase in afterload because it is already dilated from volume overload. The PAR must therefore be reduced using the usual techniques: normobaric hyperventilation, NO, prostacyclins, etc (see Table 12.11). Avoid any pulmonary vasoconstriction (hypercapnia, acidosis, hypoxia, N2O, desflurane, protamine, stress, pain, hypothermia).
- Contractility: Necessary inotropic support with beta-amines (dobutamine) and phosphodiesterase-3 inhibitors (amrinone, milrinone) or levosimendan. If necessary, non-closure of the pericardium and sternum to avoid compression of the dilated RV and reduce interventricular interference with the LV.
- Frequency: tachycardia is beneficial: reduction in RV end-diastolic volume; maintenance of sinus rhythm.
- IPPV: IPPV and PEEP can cause right-sided decompensation if the pressures generated are excessive; hyperventilation required to reduce PAR should be at the lowest possible mean intrathoracic pressure (Pit mean )
| Hemodynamics sought in severe tricuspid insufficiency |
|
High right preload in HRV Low PAR - RV inotropic support - Low Pit |
Ventilation and right heart dysfunction
The effects of positive pressure ventilation (PPV) are detrimental to right ventricular function: reduced preload, impaired diastolic expansion, increased afterload. Restoration of RV performance generally requires an increase in circulating volume and a reduction in ventilatory pressures (low Pitmean). In cases of right-sided failure, the situation can obviously be very difficult. However, three different cases can be distinguished, all of which are associated with tricuspid regurgitation.
- Right ventricular failure due to myocardial dysfunction (right myocardial infarction, arrhythmogenic RV cardiomyopathy, acute right heart failure after ECC);
- Right ventricular failure due to acute excess afterload (pulmonary embolism, protamine);
- Right ventricular hypertrophy in chronic PHT: mitral disease, LV failure, severe COPD, Eisenmenger's syndrome, etc.
In the first two cases, the aim is fourfold: 1) to maintain optimal RV preload, 2) to reduce afterload as much as possible, 3) to support function with an inotropic agent (dobutamine, milrinone) and 4) to ensure maximum right coronary perfusion (norepinephrine to increase aortic pressure). Filling is adapted to the situation: the CVP must be sufficient, but it must be reduced if the RV is congestive (nitroglycerine, diuretic, anti-Trendelenburg position). Tolerance to positive pressure ventilation is very low in cases of RV dysfunction, as the failing ventricle may decompensate to the increase in afterload represented by the IPPV. In the case of failure due to excessive afterload, the situation is more complex because the IPPV adds little to the already very high afterload, but this small increase may occasionally be enough to trigger decompensation. These two categories of patients benefit more from spontaneous breathing; if it is required, mechanical ventilation must keep Pitmean as low as possible by adjusting the ventilator and avoiding PEEP. Tolerance to IPPV can be tested preoperatively by having the patient perform a Valsalva manoeuvre and observing the effect on blood pressure ( arterial catheter ).
In the third case, chronic pulmonary vascular pathology has led to LV hypertrophy and the situation is very different. The hypertrophied RV functions like the LV: its flow depends on preload (rectified Starling curve); it needs a high CVP. As far as ventilation is concerned, four phenomena come into play [4,6].
- In COPD, ventilatory pressures are poorly transmitted by the poorly compliant lung parenchyma; the generated Pit is lower than the ventilatory pressure and the right afterload is less increased than the respiratory pressures would suggest.
- In chronic pulmonary hypertension (mean PAP > 30 mmHg), the increase in Pit due to LPI represents a small fraction of the normal pulmonary perfusion pressure; for example, a Pitmean of 12 mmHg represents only a quarter of the increase in ejection impedance of the RV when the latter is working in chronic PHT of 70/35 mmHg, whereas it doubles the afterload in a normal RV. PPV is better tolerated in PHT than in normal PAP.
- In chronic PAH, the arterial walls are thickened, muscularised and fibrosed; they resist compression by ventilatory pressure, which is not the case in normal pulmonary arteries.
- Even in the presence of PHT, the reactivity of the arterioles to acidosis and hypocapnia persists; PAP can still increase during hypoventilation. PPV can therefore reduce pulmonary resistance by hyperventilation (hyperoxia, hypocarbia and alkalosis).
Patients with chronic Pulmonary Arterial Hypertension (PHT) therefore clearly benefit from positive pressure ventilation.
Anaesthesia technique
The type of anaesthesia depends largely on the function of the ventricles and any left-sided pathology. These are recommendations for anaesthesia for isolated severe TI; if TI is associated with mitral valve disease, the latter will dictate the anaesthetic technique.
- Induction: choose agents with no significant effect on RV function or PAR.
- Etomidate: does not alter preload, afterload or contractility of the RV.
- Midazolam: adequate, reduction of central sympathetic tone reduces PAR.
- Propofol: reduces preload more than afterload; can be used in stable patients in appropriate doses and slowly, compensating with volume; should be avoided in cases of RV failure.
- Fentanils: slow administration due to bradycardia.
- Thiopental, ketamine: the increase in SAR (ketamine) and PAR (thiopental, ketamine) is prohibitive; the negative inotropic effect of thiopental is excessive.
- Maintain anaesthesia: try to reduce PAR and maintain SAR; avoid bradycardia.
- Sevoflurane: little effect on SAR and PAR.
- Perfusion of propofol or midazolam: adjust doses to ensure haemodynamic stability.
- Desflurane: not recommended (↑ PAR).
- Low Pit ventilation, no PEEP; find the best compromise between the hyperventilation required to lower PAR and the lowest mean Pit possible; in HRV and chronic PHT, increasing Pit has no significant effect on RV afterload.
- Systemic hypotension: vasopressor α .
- Support the contractile performance of the RV.
- Pulmonary vasodilatation (normobaric hyperventilation, NO•, prostacyclins, magnesium, deep anaesthesia, fentanyl).
- Preload optimisation.
- Inotropic support (dobutamine, milrinone ± adrenaline).
- Maintenance of coronary perfusion and septal balance (norepinephrine).
- Monitoring: The Swan-Ganz pulmonary catheter, although useful for measuring PAP, gives incorrect CO values distorted by the retrograde flow of the TI; it is very difficult to float through the tricuspid valve because of regurgitation and hinders the surgeon during the procedure (the catheter must be removed in the SVC); it cannot be used for valve replacement. Alternatives:
- Measurement of SV and CO using a PiCCO or similar system.
- Catheter surgically placed in the RV outflow tract if PAP measurement is required.
- TEE monitoring:
- Size and function of RV, degree of dilatation, measurement of PAP by Vmax of TI.
- Monitoring of LV function, position of the interventricular septum.
- Assessment of blood volume.
- Evaluation and mechanism of IT.
- Post-ECC: results of the procedure.
ECC and Post-ECC
The correction of tricuspid regurgitation corresponds to the loss of a pressure valve for the RV. The latter must push against the PAR, which means an increase in its afterload. Right-sided dysfunction is common at the end of ECC and its persistence is associated with a poor prognosis. From this point of view, the residual leakage of a prosthesis is a haemodynamic advantage, whereas the tightness of a prosthesis represents an increase in work. On the other hand, the prosthesis has a gradient that limits the end-diastolic preload and a stiffness that counteracts the peristaltic contraction of the RV. When a tricuspid prosthesis is fitted, the following values are considered indicative of stenosis Vmax of E flow > 1.7 m/s, mean gradient > 6 mmHg, half-pressure time > 230 ms [7]. Treatment of right-sided failure (see Table 12.6) :
- Pulmonary vasodilation (normobaric hyperventilation, NO•, prostacyclins, magnesium);
- Preload optimisation;
- Inotropic support (dobutamine, milrinone ± epinephrine, levosimendan);
- Maintenance of coronary perfusion and interventricular septal position (norepinephrine);
- No closure of the pericardium and sternum ; possibly after 48-72 hours.
Carcinoid syndrome
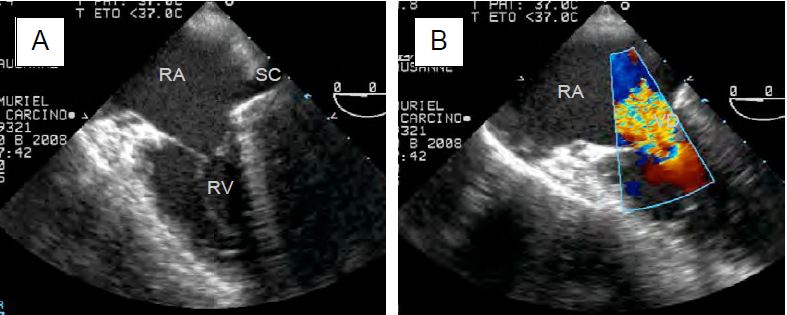
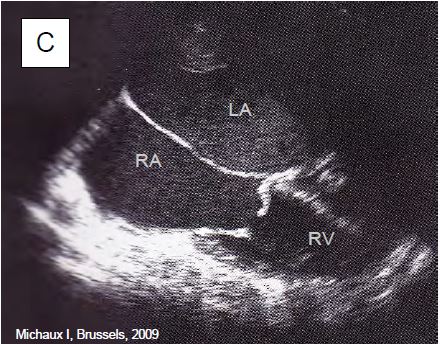
Neuroendocrine tumours (NETs) are rare (2-5 cases per 100,000 population); they develop mainly in the digestive tract and manifest as secretions of serotonin (5-hydroxytryptamine) and vasodilating agents such as VIP (vasoactive intestinal peptide). They are characterised by episodes of vasoplegia, flushing, diarrhoea and bronchospasm. In the heart, which is affected in 30-50% of cases, the disease develops subendocardial fibrous thickening of the leaflets of the right valves and sometimes of the left valves in the presence of a patent foramen ovale or a right-left shunt. The most commonly affected valve is the tricuspid. By the time cardiac symptoms appear on the tricuspid and/or pulmonary valve, the tumour has metastasised to the liver and survival is only 31% at 3 years [3]. The diagnosis is made clinically and by measuring 5-hydroxyindoleacetic acid (>300 mmol/24 h in urine) and NT-proBNP. On echocardiography, the tricuspid and pulmonary valves appear thickened, rigid, retracted and poorly mobile; they are inadequate and partially stenotic (Figure 11.160).
Figure 11.160: TEE images of tricuspid lesion (TLI) in carcinoid syndrome. A: 4-cavity view (low esophageal position) of a massive TI; the leaflets are thickened, restrictive and do not coapt in systole. SC: Coronary sinus. B: Identical view with colour Doppler flow; the vena contracta is large (> 1 cm); there is a significant PISA. C: Tricuspid stenosis in carcinoid syndrome; the restrictive diastolic orifice has a domed appearance; the leaflets are thickened and connected; the RV is small while the RA is dilated.
Specific treatment is based on somatostatin analogues (octreotide and lanreotide), which improve symptoms in two thirds of cases. Embolization or surgical resection (debulking) of liver metastases greatly improves the situation, but at the cost of high operative risk, significant bleeding and postoperative liver failure. Cardiac surgery allows tricuspid and/or pulmonary valve replacement, but operative mortality is high (18%) and survival is only 69% at 1 year and 24% at 10 years [1,2,5]. If necessary, the operation can be completed with mitral or aortic replacement with closure of the PFO. Bioprostheses are preferred to avoid anticoagulation because of liver problems and the possibility of subsequent surgery.
There are two major risks associated with the procedure: 1) serotonin crisis and 2) right-sided failure. As both are characterised by severe hypotension, TEE and a pulmonary catheter are required to make the differential diagnosis. Severe serotonin release with hypotension, tachycardia, arrhythmias and bronchospasm may be caused by surgical stress, histamine releasers, catecholamines and morphine. Hyperglycaemia is usually marked. Selective treatment of perioperative carcinoid crisis consists of administration of a somatostatin analogue, such as octreotide or lanreotide, combined with a non-catecholaminergic alpha vasoconstrictor (phenylephrine infusion) or an anti-NO (methylene blue). Octreotide (Sandostatin® ) is given as an infusion (50-100 mcg/h, maximum 300 mcg/h) starting 12 hours before the procedure and continuously during the procedure; boluses of 100-200 mcg can be given as required [1,3]. The safest anaesthetic agents are etomidate, midazolam, fentanyl, sevoflurane, vecuronium and rocuronium. If inotropic support is required because of RV failure, milrinone is the preferred choice.
| Anaesthesia and tricuspid insufficiency |
| In primary TI, anaesthesia is administered according to the performance of the RV. In secondary TI, anaesthesia is administered according to the pathology of the left heart.
Mechanical ventilation in the presence of RV dysfunction can be divided into 3 different situations: - Primary RV failure: IPPV is poorly tolerated because RV afterload must remain low - Acute increase in right afterload (pulmonary embolism): variable tolerance to IPPV - Chronic PHT with HRV: IPPV are well tolerated and beneficial Carcinoid syndrome: tricuspid and pulmonary valve involvement in 50% of cases. Features: - Massive serotonin release (vasoplegia, arrhythmias, bronchospasm) - Intervention with continuous infusion of Sandostatin® (50-300 mcg/h) + bolus 100-200 mcg - Safe drugs: etomidate, midazolam, fentanyl, sevoflurane, vecuronium, rocuronium - Avoid catecholamines |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- CASTILLO JG, FILSOUFI F, ADAMS DH, et al. Management of patients undergoing multivalvular surgery for carcinoid heart disease: the role of the anaesthetist. Br J Anaesth 2008; 101:618-26
- CONNOLLY HM, SCHAFF HV, MULLANY CJ, et al. Carcinoid heart disease: Impact of valve replacement on right ventricular function and remodeling. Circulation 2002; 106:I-51-8
- DAVAR J, CONNOLLY HM, CAPLIN ME, et al. Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors. J Am Coll Cardiol 2017; 69:1288-304
- FISCHER LG, VAN AH, BURKLE H. Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists. Anesth Analg 2003; 96:1603-16
- MOKHLES P, VAN HERWERDEN LA, DE JONG PL, et al. Carcinoid heart disease: outcomes after surgical valve replacement. Eur J Cardio-Thorac Surg 2012; 41:1278-83
- STRUMPHER J, JACONSOHN E. Pulmonary hypertension and right ventricular dysfunction: physiology and perioperative management. J Cardiothorac Vasc Anesth 2011; 25: 687-704
- ZOGHBI WA, CHAMBERS JB, DUMESNIL JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocradiogr 2009; 22:975-1014