ASDs are the second most common form of congenital heart defect after bicuspid aortic valve. Their incidence is 7% of all congenital defects and 30% of those observed in adults [9]. Five types of defects may occur in the atrial septum (Figure 15.10) [3,49].
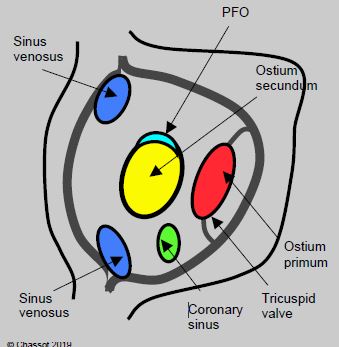
Figure 15.10: View of the atrial septum from the RA with the positioning of the various anatomical variants of atrial septal defects [11,12].

Figure 15.10: View of the atrial septum from the RA with the positioning of the various anatomical variants of atrial septal defects [11,12].
- Ostium secundum situated in the centre of the septum at the site of the fossa ovalis, accounting for 75% of cases;
- Ostium primum defect situated near the tricuspid valve, which is part of the atrioventricular canal defect (15% of cases);
- Sinus venosus situated at the origin of a vena cava (usually the SVC), often associated with partial anomalous pulmonary venous return (5-10% of cases);
- Absence of the coronary sinus roof allowing it to communicate with the LA (< 1%);
- Special case: patent foramen ovale (PFO).
The flow of a left → right (L-to-R) shunt is dependent on its size (diameter of 5 to 30 mm) and the compliance of the LV. A gradual fall in compliance in adults raises LV end-diastolic pressure and therefore LA pressure. This augments the L-to-R shunt and increases RV overload [6]. It causes volume overload on the right side and therefore dilation of the RA, RV and PA (Figure 15.11, Figure 15.12 and Figure 15.13).
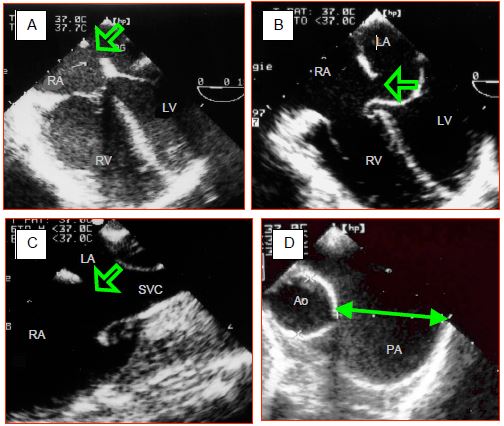
Figure 15.11: Transesophageal echocardiographic images of atrial septal defects (ASDs). A: ostium secundum-type ASD, located in the centre of the fossa ovalis. The RV is dilated. B: ostium primum-type ASD associated with an AV canal defect. The hemmed appearance of the septal leaflet of the tricuspid valve indicates the spontaneous closure of an underlying VSD. C: superior sinus venosus-type ASD located at the anastomosis of the superior vena cava (SVC). D: dilation of the pulmonary artery (PA) whose diameter measured during diastole is almost double that of the ascending aorta.
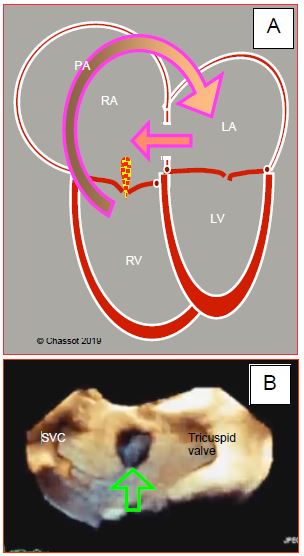
Figure 15.12: Atrial septal defect. A: Diagram showing the outline of the 4 cardiac chambers in the event of ASD with major left-to-right shunting (arrow). The RA and RV are dilated. The RV is hypertrophied. The apex of the heart is formed by the RV and not the LV. B: Three-dimensional echocardiographic view of the atrial septum from the RA with an ostium secundum-type ASD at its centre.

Figure 15.13: Transesophageal echocardiographic images of two ASDs. A: Unrestrictive, large opening between LA and RA; the flow velocity is low. B: restrictive, small opening; the flow velocity is accelerated. Despite the difference in velocity, the amount of blood shunted is larger in the first case.
The RV is dilated and hypertrophied to accommodate the volume overload. The PA diameter is larger than that of the aorta and the flow is accelerated in it. The normal ratio of 0.6 between the right and left dimensions and between PA and aorta velocity is more than doubled. An echocardiographic diagnosis is established based on a visible defect in the septum (Figure 15.11), systolic and diastolic flow between the two atria (Figure 15.13) and dilation of the right chambers (Videos).
Video: Colour flow showing the left-to-right shunt through an ostium secundum ASD; the shunt is flowing from left atrium (above on the screen) to right atrium (below).
Video: Dilatative hypertrophy of the right ventricle in a case of ASD; the right ventricle is larger than the left.

Figure 15.11: Transesophageal echocardiographic images of atrial septal defects (ASDs). A: ostium secundum-type ASD, located in the centre of the fossa ovalis. The RV is dilated. B: ostium primum-type ASD associated with an AV canal defect. The hemmed appearance of the septal leaflet of the tricuspid valve indicates the spontaneous closure of an underlying VSD. C: superior sinus venosus-type ASD located at the anastomosis of the superior vena cava (SVC). D: dilation of the pulmonary artery (PA) whose diameter measured during diastole is almost double that of the ascending aorta.

Figure 15.12: Atrial septal defect. A: Diagram showing the outline of the 4 cardiac chambers in the event of ASD with major left-to-right shunting (arrow). The RA and RV are dilated. The RV is hypertrophied. The apex of the heart is formed by the RV and not the LV. B: Three-dimensional echocardiographic view of the atrial septum from the RA with an ostium secundum-type ASD at its centre.

Figure 15.13: Transesophageal echocardiographic images of two ASDs. A: Unrestrictive, large opening between LA and RA; the flow velocity is low. B: restrictive, small opening; the flow velocity is accelerated. Despite the difference in velocity, the amount of blood shunted is larger in the first case.
The RV is dilated and hypertrophied to accommodate the volume overload. The PA diameter is larger than that of the aorta and the flow is accelerated in it. The normal ratio of 0.6 between the right and left dimensions and between PA and aorta velocity is more than doubled. An echocardiographic diagnosis is established based on a visible defect in the septum (Figure 15.11), systolic and diastolic flow between the two atria (Figure 15.13) and dilation of the right chambers (Videos).
Video: Colour flow showing the left-to-right shunt through an ostium secundum ASD; the shunt is flowing from left atrium (above on the screen) to right atrium (below).
Video: Dilatative hypertrophy of the right ventricle in a case of ASD; the right ventricle is larger than the left.
The flow through the shunt exhibits a typical biphasic shape (Vmax 0.5-1.5 m/s), with an end systolic - early diastolic peak corresponding to the v-wave and a peak during atrial contraction corresponding to the a-wave [5,11]. Between the two of them, there are two periods during which the flow rate slows or even inverts – these correspond to the pressure drops “x” and “y” (Figure 15.14) [24,32].
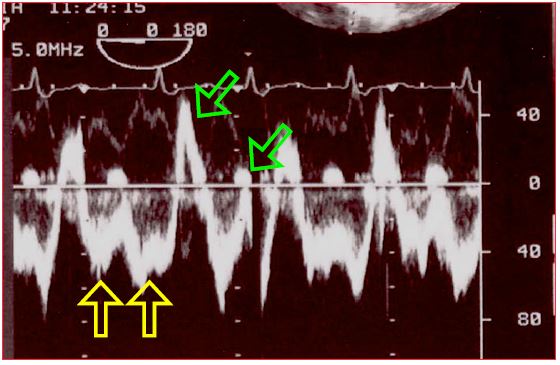
Figure 15.14: Spectral Doppler images of the flow through an ASD. The shunt is predominantly left-to-right. The main flow is below the baseline since it moves away from the echocardiographic transducer located in the oesophagus, from the LA to the RA. It consists of two components (yellow arrows). Two minor components in the opposite direction (right-to-left) are also visible above the baseline (green arrows).
This morphology relates to pressure variations in the atria during the cardiac cycle [30]. Indeed, the amplitude of pressure variations is greater in the LA than in the RA – pressure is therefore higher on the left side than on the right side during pressure peaks, but lower during pressure troughs, enabling the flow to invert at this point (Figure 15.15) [5].
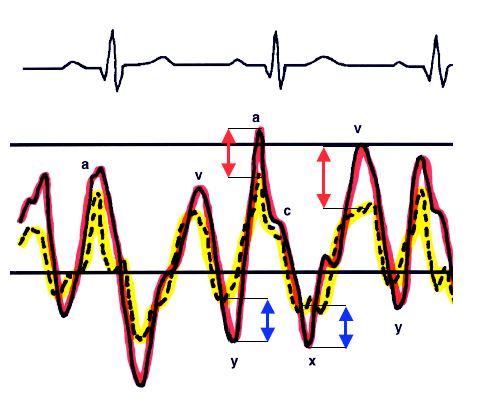
Figure 15.15: Representation of the pressure curves of the LA (red) and RA (yellow). Pressure variations are more significant on the left side. Consequently, PLA is higher than PRA during pressure peaks, but lower during pressure troughs. The flow is therefore left-to-right during the a- and v-waves (red arrows), but right-to-left during the x and y descents (blue arrows) [30].
The most significant inversion occurs during systole since the descent of the mitral annulus causes a sudden increase in LA volume and a major fall in its pressure. The shunt's R-to-L component intensifies if RA filling increases due to a drop in intrathoracic pressure (relaxation post-Valsalva or Müller's manoeuvre) – the same applies if RA pressure increases due to rising RV afterload and filling pressure e.g. during a Valsalva manoeuvre, inspiration of IPPV or with PEEP (Figure 15.16) [24]. Therefore, even a predominantly L-to-R shunt may cause paradoxical embolism as a result of a Valsalva manoeuvre or excessive PEEP (Figure 15.17) (Video). This requires the anaesthetist to “hunt for bubbles” in all venous lines to avoid cerebral or coronary gas embolism.
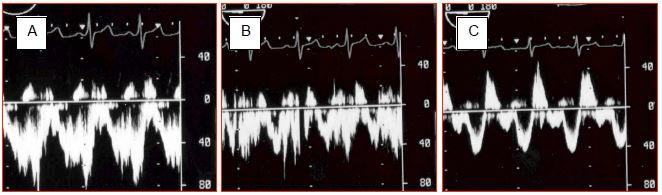
Figure 15.16: Impact of ventilation on ASD flow. During apnoea (A), the flow is virtually exclusively left-to-right. During IPPV (B), the velocity of the left-to-right flow decreases. Adding PEEP 10 cm H2O (C) further reduces the left-to-right component and increases the right-to-left component. Despite adequate ventilation, arterial oxygen saturation will fall.
Video: Spontaneous flow of microbubbles from RA into LA in a case of ostium secundum ASD.
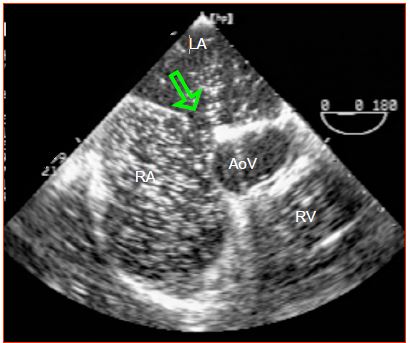
Figure 15.17: If pressure in the RA is temporarily higher than that in the LA, the flow inverts through the ASD (arrow). Paradoxical embolism may occur at this time, as indicated by the movement of microbubbles from the RA to the LA in the presence of PEEP. VAo: aortic valve.
Small ASDs may remain asymptomatic for a very long time, the only sign being a high-flow grade 3/6 systolic murmur through the pulmonary valve that can be heard at the left upper border of the sternum, and a split second heart sound. The ECG shows a right axis deviation and the chest X-ray reveals enlargement of the right chambers and the border of the PA. However, adults most commonly present with exertional dyspnoea, atrial arrhythmias and periods of right-sided decompensation. ASDs associated with an increase in pulmonary blood flow of over 50% (Qp: Qs > 1.5), paradoxical embolism, and right-side dilation must be closed to prevent arrhythmias (atrial tachyarrhythmias) and RV dilation that leads to right-sided decompensation in the long term [18,26].
Volume overload dilates the RV and the tricuspid annulus, causing tricuspid insufficiency, which is generally grade 1-2 out of 4. If the shunt is very large, it causes moderate pulmonary arterial hypertension (PAH) (rarely > 500 dynes•s•cm-5), hypertrophy, and subsequently RV failure. In this case, the shunt flow decreases as well as the pressure gradient between the LA and RA – the shunt becomes bidirectional. It may even invert and the patient becomes cyanotic. However, Eisenmenger’s syndrome (fixed PAH) is rare, occurring in less than 5% of patients aged over 40 years [3,9]. Mitral insufficiency occurs in 15% of unoperated cases [31].
Surgery is indicated in the event of: a large L-to-R shunt (Qp/Qs ≥ 1.5:1), a dilated RV, dyspnoea, and/or a medical history of paradoxical embolism [3,45]. Due to the high risk of PAH, surgery is imperative in cases of sinus venosus combined with anomalous pulmonary venous return regardless of its size [6].
Central ASDs (ostium secundum or PFO), of under 40 mm surrounded by appropriately-sized borders (≥ 5 mm) are occluded by an umbrella-type prosthesis, which is fitted percutaneously (Amplatzer™, PFO Star™, Helex™ occluders, etc.), generally with transesophageal echocardiography monitoring [3,6,13,43,49]; Such implantations are followed by dual antiplatelet therapy for 6 months and aspirin for life. Large ASDs or ASDs of a different type than ostium secundum (ostium primum, sinus venosus) are closed surgically (autologous pericardial patch or direct closure). The surgical mortality rate is < 1% [3]. An optimal outcome is achieved by closing the ASD before the age of 25 years. However, sequelae can only be avoided by performing an operation before the age of 5 years. In patients aged > 60 years, the risk of complications is higher (12-23%), but surgery is always beneficial for the RV, even though it is no longer possible to restore its normal size and function [22]. If PAH is present, closure of an ASD is only indicated if the shunt is mostly L-to-R, if sPAP is < 0.5 sAP, and if the PVR/SVR ratio is < 0.6, which is very often the case [3,43,45.49]. Closure is contra-indicated if the shunt is R-to-L or if PVR > 0.65 SVR [45].
Patent foramen ovale (PFO)
PFO is frequently detected in the normal population. Its incidence varies from 5-17% with two-dimensional echocardiography, to 24% at autopsy or to 27% on direct perioperative inspection in which it presents as a slit measuring approximately 20 mm [2,4,25,28,42]. It is commonly associated with an elongation of the fossa ovalis membrane forming a floating membrane (63% of cases) or an aneurysm of the atrial septum (42% of cases) [1]. Normally, the fossa ovalis membrane fuses with the fibromuscular part of the interatrial septum in the first year of life. If this fusion does not occur, the membrane is simply attached due to the higher pressure in the LA. This generally allows a small crossing in the form of a permanent yet minimal L-to-R shunt. However, if RA pressure temporarily exceeds LA pressure (Valsalva manoeuvre, PEEP, pulmonary embolism), the PFO opens due to shifting of the membrane into the LA and thus constitutes a R-to-L shunt entailing a risk of arterial desaturation and systemic embolisation (Video) [47].
Video: A thrombus is trapped in the patent foramen ovale during an episode of pulmonary embolism; the pressure overload on the RV has reversed the flow through PFO into a R-to-L shunt.
PFO is diagnosed with echocardiography by the presence of a colour flow (Figure 15.18) and by a microbubble test (Figure 15.19) [2,33].
- A colour Doppler reveals a L → R shunt in 80% of PFO cases;
- A microbubble test is used to detect whether the shunt has a R → L component (Video).
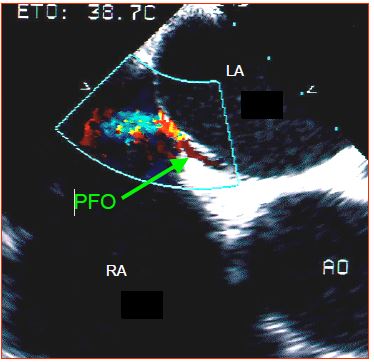
Figure 15.18: Patent foramen ovale (PFO). TEE image of the colour flow passing between the fossa ovalis membrane and the fibromuscular part of the atrial septum. Ao: aortic valve.
Video: Microbubble test; microbubbles flow from RA to LA when RAP is higher than LAP during the cardiac cycle.
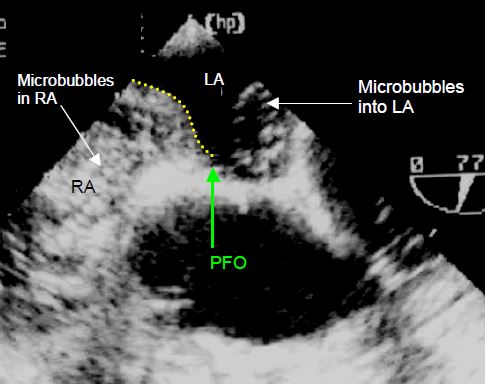
Figure 15.19: Microbubble test. Image of microbubbles moving from the RA to the LA. The RA (and RV) is filled with contrast agent. On each cardiac cycle, a surge of microbubbles is ejected into the LA through the PFO. The dotted yellow line shows the position of the atrial septum.
The microbubble test is performed by rapid injection, preferably through a central line, of 10 ml NaCl 0.9% in which microbubbles have been created by cavitation between two syringes. It demonstrates an opacification of the RA and the immediate appearance of microbubbles in the LA and aorta. If the microbubbles reach the LA more than 5 cardiac cycles after their appearance in the RA, this is probably due to transpulmonary crossing. Two different manoeuvres are performed to encourage a R-to-L crossing [19].
- Increasing pressure in the RA by increasing RV afterload through high PEEP (20-25 cm H2O) or a Valsalva manoeuvre;
- Increasing RA volume by abruptly lowering the pressure in the airways. This increases the venous return to the RA, but temporarily impedes venous return to the LA.
These two provocation manoeuvres raise the diagnosis rate to 92% of PFOs [1,2].
Like ASDs, PFOs may cause paradoxical embolisms, which are much rarer than the incidence of the lesion itself, since they occur in only 1-2% of cases [7]. These embolisms can sometimes lead to strokes [29]. They probably occur during Valsalva manoeuvres, in the event of acute pulmonary hypertension following a massive pulmonary embolism, or in the event of gas embolisation, for instance during coelioscopy, neurosurgical procedures in a sitting position (12-39% of cases), or decompression after scuba diving [8,16]. However, amateur scuba diving is not considered to be an indication for a systematic search for a PFO or its closure [48]. The chance of paradoxical embolisms is increased by: the presence of an atrial septal aneurysm; persistence of the Eustachian membrane diverting blood from the IVC to the PFO; and atrial arrhythmias [23].
The major problem with PFOs is their level of association with embolic strokes. Many studies demonstrate that the lesion is significantly correlated with cryptogenic strokes (OR 3.0 - 3.7) [7,20,39]. For example, patients with PFOs who experience pulmonary embolisms more frequently suffer silent cerebral infarction (OR 34.9) [14] and their mortality is three times higher than that of patients without PFOs [27]. This relationship is more pronounced in cases where the PFO is associated with an atrial septal aneurysm (OR 15.6) [39]. A relationship also appears to exist with refractory migraine with aura [23]. However, the prevalence of PFO in cases of ischaemic strokes that have no clear link to embolism is 35-40% versus 20-25% in the general population. This slight difference allows uncertainty to persist over a possible fortuitous relationship [21].
There is still no clear solution to the problem of whether or not to close a PFO after an embolic stroke. Following several studies demonstrating equal results in terms of stroke relapse between percutaneous closure by an umbrella or medical treatment (anticoagulant or anti-platelet agent) [17,35], several recent randomised studies appear to demonstrate the superiority of surgical treatment in preventing relapses: hazard ratio 0.49 [10], 0.38 [41], 0.23 [44] or 0.03 [34] in favour of invasive therapy. While the level of risk associated with the procedure is low (1.5-5.9%) [10,34], the incidence of AF after closure is 4.6-6.6% [34,44]. The more decisive results in the latest trials are probably due to stricter patient selection (wide PFO with septal aneurysm, no intercurrent causes of stroke, age < 60 years, early CT-scan or MRI with images of non-lacunar ischaemic stroke), and the presence of an antiplatelet and/or anticoagulant treatment. Currently, PFO closure by catheterisation is regarded as the first-choice treatment for preventing stroke relapse if there is no evidence of any other aetiology for the stroke and the PFO is major [21,38].
In cases where a PFO is discovered fortuitously during cardiac surgery, the question arises of whether to close it during CPB, which requires double venous cannulation and extended surgery. A survey has demonstrated that 28% of surgeons take this step systematically, while the majority only close the PFO if it is large, if the patient as a medical history of arterial embolism, or if pressure in the RA is high [46]. Perioperative closure of an asymptomatic PFO does not change postoperative morbimortality. On the contrary, it appears to increase the risk of stroke (OR 2.47) [28]. Moreover, the guidelines remain highly conservative regarding the indication to occlude a PFO and only recommend such a step in the event of a paradoxical embolism causing a cryptogenic stroke or repeated arterial occlusions [6,15,36,37]. The clinical data are indeed insufficiently robust to determine a direct causal relationship between PFO and stroke, and closure of a PFO does not preclude stroke or paradoxical embolism relapse. If an asymptomatic PFO is discovered fortuitously by perioperative TEE, it should therefore be closed “en-passant” only if some conditions are fulfilled [40].
Like ASDs, PFOs may cause paradoxical embolisms, which are much rarer than the incidence of the lesion itself, since they occur in only 1-2% of cases [7]. These embolisms can sometimes lead to strokes [29]. They probably occur during Valsalva manoeuvres, in the event of acute pulmonary hypertension following a massive pulmonary embolism, or in the event of gas embolisation, for instance during coelioscopy, neurosurgical procedures in a sitting position (12-39% of cases), or decompression after scuba diving [8,16]. However, amateur scuba diving is not considered to be an indication for a systematic search for a PFO or its closure [48]. The chance of paradoxical embolisms is increased by: the presence of an atrial septal aneurysm; persistence of the Eustachian membrane diverting blood from the IVC to the PFO; and atrial arrhythmias [23].
The major problem with PFOs is their level of association with embolic strokes. Many studies demonstrate that the lesion is significantly correlated with cryptogenic strokes (OR 3.0 - 3.7) [7,20,39]. For example, patients with PFOs who experience pulmonary embolisms more frequently suffer silent cerebral infarction (OR 34.9) [14] and their mortality is three times higher than that of patients without PFOs [27]. This relationship is more pronounced in cases where the PFO is associated with an atrial septal aneurysm (OR 15.6) [39]. A relationship also appears to exist with refractory migraine with aura [23]. However, the prevalence of PFO in cases of ischaemic strokes that have no clear link to embolism is 35-40% versus 20-25% in the general population. This slight difference allows uncertainty to persist over a possible fortuitous relationship [21].
There is still no clear solution to the problem of whether or not to close a PFO after an embolic stroke. Following several studies demonstrating equal results in terms of stroke relapse between percutaneous closure by an umbrella or medical treatment (anticoagulant or anti-platelet agent) [17,35], several recent randomised studies appear to demonstrate the superiority of surgical treatment in preventing relapses: hazard ratio 0.49 [10], 0.38 [41], 0.23 [44] or 0.03 [34] in favour of invasive therapy. While the level of risk associated with the procedure is low (1.5-5.9%) [10,34], the incidence of AF after closure is 4.6-6.6% [34,44]. The more decisive results in the latest trials are probably due to stricter patient selection (wide PFO with septal aneurysm, no intercurrent causes of stroke, age < 60 years, early CT-scan or MRI with images of non-lacunar ischaemic stroke), and the presence of an antiplatelet and/or anticoagulant treatment. Currently, PFO closure by catheterisation is regarded as the first-choice treatment for preventing stroke relapse if there is no evidence of any other aetiology for the stroke and the PFO is major [21,38].
In cases where a PFO is discovered fortuitously during cardiac surgery, the question arises of whether to close it during CPB, which requires double venous cannulation and extended surgery. A survey has demonstrated that 28% of surgeons take this step systematically, while the majority only close the PFO if it is large, if the patient as a medical history of arterial embolism, or if pressure in the RA is high [46]. Perioperative closure of an asymptomatic PFO does not change postoperative morbimortality. On the contrary, it appears to increase the risk of stroke (OR 2.47) [28]. Moreover, the guidelines remain highly conservative regarding the indication to occlude a PFO and only recommend such a step in the event of a paradoxical embolism causing a cryptogenic stroke or repeated arterial occlusions [6,15,36,37]. The clinical data are indeed insufficiently robust to determine a direct causal relationship between PFO and stroke, and closure of a PFO does not preclude stroke or paradoxical embolism relapse. If an asymptomatic PFO is discovered fortuitously by perioperative TEE, it should therefore be closed “en-passant” only if some conditions are fulfilled [40].
- The operation already involves an atriotomy;
- PFO is large (wide colour flow) or the membrane is too short to occlude it;
- The shunt is bidirectional;
- PFA is combined with a septal aneurysm;
- There is a risk of RAP exceeding LAP (right-sided failure, pulmonary hypertension, COPD, fall in LAP after correction of mitral valvular disease or after implanting a left ventricular assist device);
- Donor heart for transplantation, implantation of a left ventricular assist device.
Anaesthesia in the event of ASDs
The impact of ASDs on the course of anaesthesia depends on the size of the shunt, its predominant direction, and the presence of any pulmonary arterial hypertension (PAH) (Table 15.3) [12].
- Haemodynamic management is aimed at reducing the extent of shunting. This is achieved by lowering systemic vascular resistance (SVR) and slightly increasing pulmonary vascular resistance (PVR) (Figure 15.20). Provided that PAH is not present, this is achieved by systemic vasodilation and slight hypoventilation (FiO2 0.3, PaCO2 45 mmHg).
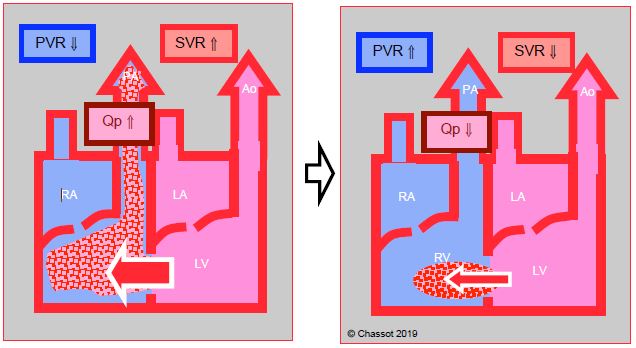
Figure 15.20: Left → right shunt. This is increased if SVR rises and PVR falls, in which case pulmonary blood flow (Qp) is excessive. The shunt is reduced if SVR falls and PVR rises. This is the goal pursued by anaesthesia. Since LV compliance decreases with age, LV end-diastolic pressure tends to increase, raising LA pressure and reinforcing L-to-R shunting.
- Positive pressure ventilation (IPPV) and particularly PEEP raise RV afterload, impeding the shunt's L-to-R component while increasing the R-to-L component. In patients with partially bidirectional shunting, it may cause arterial desaturation. Low-pressure ventilation is recommended with FiO2 of 0.3, while maintaining moderate hypercapnia.
- In the event of pulmonary arterial hypertension (PAH), positive pressure ventilation further increases right-sided afterload. However, this rise in pressure is small compared to resting pulmonary artery pressure. It represents an even smaller increase in afterload than in normal individuals. Moreover, with controlled ventilation it is possible to lower PVR by hyperventilation and hypocapnia (see Pulmonary Hypertension).
- Systemic arterial vasodilation reduces left-sided pressure and lowers the volume of the L-to-R shunt. From this perspective, isoflurane and neuraxial blockade are appropriate choices. However, if the shunt is already bidirectional because right-sided overload has induced PAH, the shunt’s R-to-L component increases along with arterial desaturation.
- Hypovolaemia is poorly tolerated, since the patient needs high circulating volume to maintain his/her systemic flow, given that some of the volume is inevitably sequestrated by the shunt between the RA and the LA via the low-pressure pulmonary circuit. This is particularly true if SVR is high.
- Measurement of CVP is a poor indicator of preload, since RAP is high due to the shunt and any tricuspid insufficiency.
- The RV requires a particularly high preload if it is hypertrophied. Right ventricular function may be improved by increasing contractility and heart rate (dobutamine, milrinone).
- Any rise in pressure in the RA increases the risk of paradoxical embolism – this may occur following peripheral thromboembolism or accidental injection of air or particles through venous lines. Any air bubbles must be meticulously eliminated from infusions.
- LV diastolic dysfunction, which is common in adults, raises LV end-diastolic pressure and therefore LA pressure. This augments the L-to-R shunt and increases RV overload [6].
- Closing an ASD renders the patient suddenly hypervolaemic, since the volume previously diverted to the pulmonary circuit by the shunt, which had only circulated between the RA and LA, is added to the systemic circulating volume.
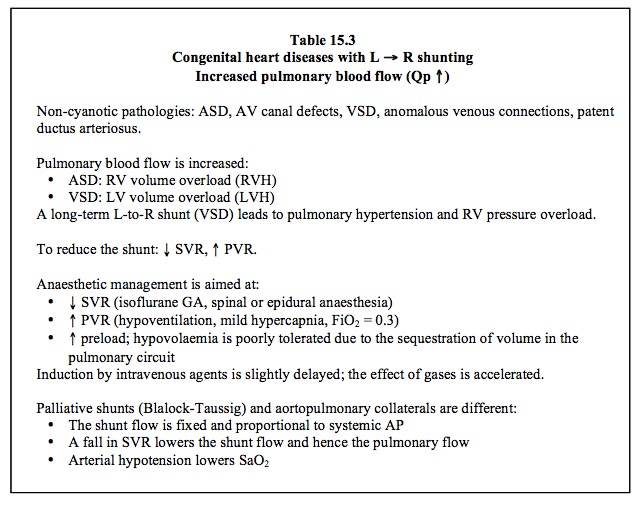
| Atrial septal defects (ASDs) |
|
Characteristics:
- Non-cyanotic L → R shunt with increased pulmonary blood flow (Qp ↑, Qp:Qs > 1.5) - Small R → L component possible (risk ↑ if PAH or ↑ RAP) - Dilation of receiving chambers: RA, RV, PA - Volume overload for the RV - Pulmonary hypertension uncommon (< 10% of adults: PVR 300-500 dynes•cm•s-5) - Surgical indications: Qp/Qs > 1.5, RV dilation, dyspnoea, arrhythmia Indications for closing a PFO: paradoxical embolism causing a stroke or arterial occlusions During cardiac surgery: bidirectional PFO, atriotomy and risk of RAP > LAP. SVR must be lowered and PVR increased in order to reduce shunting. Anaesthesia recommendations: - GA with isoflurane - Neuraxial blockade (spinal anaesthesia, epidural) - Ventilation: FiO2 0.3, normocapnia or slight hypercapnia, low ventil P, no PEEP - Hypovolaemia poorly tolerated due to the sequestration of volume in the pulmonary circulation |
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
- AGOUSTIDES JG, WEISS SJ, OCHROCH AE, et al. Analysis of the interatrial septum by transesophageal echocardiography in adult cardiac surgical patients: Anatomic variants and correlation with patent foramen ovale. J Cardiothorac Vasc Anesth 2005; 19:146-9
- AGOUSTIDES JG, WEISS SJ, WEINER J, et al. Diagnosis of patent foramen ovale with multiplane transesophageal echocardiography in adult cardiac surgical patients. J Cardiothorac Vasc Anesth 2004; 18:725-30
- BAUMGARTNER H, BONHOEFFER P, DE GROOT NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915-57
- BERKOMPAS DC, SAGAR KB. Accuracy of color Doppler trasesophageal echocardiography for diagnosis of patent foramen ovale. J Am Soc Echocardiogr 1994; 7:253-6
- BETTEX D, CHASSOT PG. Malformations congénitales de l'adulte (Congenital malformations in adults) In: BETTEX D, CHASSOT PG. Echocardiographie transoesophagienne en anesthésie – réanimation (Transesophageal echocardiography in anaesthesia/intensive care) Paris: Pradel-Masson, Williams & Wilkins, 1997, 171-87
- BHATT AB, FOSTER E, KUEHL K, et al. Congenital heart disease in older adults. A Scientific Statement from the American Heart Association. Circulation 2015; 131:1884-931
- BOGOUSSLAVSKI J, GARAZI S, JEANRENAUD X, et al. Stroke recurrence in patients with patent foramen ovale: the Lausanne study. Neurology 1996; 46:1301-5
- BOVE AA. Risk of decompression sickness with patent foramen ovale. Undersea Hyperb Med 1998; 25:175-8
- BRECKER SJD, REDINGTON A, SHORE D, OLDERSHAW P. Atrial septal defects. In: REDINGTON A, ed. Congenital heart disease in adults. A practical guide. London: WB Saunders Co Ltd, 1994, 104-10
- CARROLL JD, SAVER JL, THALER DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368:1092-100
- CHASSOT PG, BETTEX D. Perioperative transoesophageal echocardiography in adult congenital heart disease. In: POELAERT J, SKARVAN K. Transoesophageal echocardiography in anaesthesia. London, BMJ Book, 2004
- CHASSOT PG, BETTEX DA. Anesthesia and adult congenital heart disease. J Cardiothorac Vasc Anesth 2006; 20:414-37
- CHESSA M, CARMINATI M, BUTERA G, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002; 39:1061-5
- CLERGEAU MR, HAMON M, MORELLO R, et al. Silent cerebral infarcts in patients with pulmonary embolism and a patent foramen ovale. A prospective diffusion-weighed MRI study. Stroke 2009; 40:3758-62
- ESO – European Stroke Organisation. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25:457-507
- FAHTI AR, ESHTEHARDI P, MEIER B. Patent foramen ovale and neurosurgery in sitting position: a systematic review. Br J Anaesth 2009; 102:588-96
- FURLAN AJ, REISMAN M, MASSARO J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366:991-9
- GATZOULIS MA, FREEMAN MA, SIU SC, et al. Atrial arrhythmia after surgical closure of atrial septal defect in adults. N Engl J Med 1999; 340:839-44
- GREIM CA, TRAUTNER H, KRAMER K, et al. The detection of interatrial flow patency in awake and anesthetized patients: A comparative study using transnasal echocardiography. Anesth Analg 2001; 92:1111-6
- HANDKE M, HARLOFF A, OLSCHEWSKI M, et al. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007; 357:2262-8
- HANKEY GJ, McQUILLAN BM. Patent foramen ovale closure. The pendulum swings. Circulation 2018; 137:1991-3
- HANNINEN M, KMET A, TAYLOR DA; et al. Atrial septal defect closure in the elderly is associated with excellent quality of life, functional improvement, and ventricular remodelling. Can J Cardiol 2011; 27:698-704
- HORTON SC, BUNCH TJ. Patent foramen ovale and stroke. Mayo Clin Proc 2004; 79:79-88
- JAFFE RA, PINTO FJ, SCHNITTGER I, et al. Aspects of mechanical ventilation affecting interatrial shunt flow during general anesthesia. Anesth Analg 1992; 75:484-8
- KONSTADT SN, LOUIE EK, BLACK S. Intraoperative detection of foramen ovale by transesophageal echocardiography. Anesthesiology 1994; 74:212-6
- KONSTANTINIDES PJ, ANDERSON ME, ROTTMAN JN, et al. A comparison of surgical and medical therapy for atrial septal defects in adults. N Engl J Med 1995; 333:469-73
- KONSTANTINIDES S, GEIBEL A, KASPER W, et al. Patent foramen ovale is an important predictor of adverse outcome in patients with major pulmonary embolism. Circulation 1998; 97:1946-51
- KRASUSKI RA, HART SA, ALLEN D, et al. Prevalence and repair of intraoperatively diagnosed patent foramen ovale and association with perioperative outcomes and long-term survival. JAMA 2009; 302:290-7
- LECHAT P, MAS JL, LASCAULT G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318:1148-52
- LEVIN AR. Atrial pressure-flow dynamics in atrial septal defects (secundum type). Circulation 1968; 37:476-9
- LIBERTHSON RR, BOUCHER CA, FALLON JT, et al. Severe mitral regurgitation: A common occurence in the aging patient with secundum atrial septal defect. Clin Cardiol 1981; 4:229-32
- LIN FC, FU M, YEH SH, WU D. Doppler atrial flow patterns in patients with secundum atrial septal defects. Determinants, limitations and pitfalls. J Am Soc Echocardiogr 1988; 1:141-5
- LOUIE EK, KONSTADT SN, RAO TL. Transesophageal echocardiographic diagnosis of the right to left shunting across the foramen ovale in adults without prior stroke. J Am Coll Cardiol 1993; 21:1231-7
- MAS JL, DERUMEAUX G, GUILLON B, et al. Patent foramen ovale closure or anticoagulation vs antiplatelet after stroke. N Engl J Med 2017; 377:1011-21
- MEIER B, KALESAN B, MATTLE HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 203; 368:1083-91
- MEIER B, LOCK JE. Contemporary management of patent foramen ovale. Circulation 2003; 107:5-9
- MESSÉ S, GRONSETH G, KENT D, et al. Practice advisory: recurrent stroke with patent foramen ovale (update of practice parameters): report of the Guideline Development, Dissemination, and Implementation Subcomittee of the American Academy of Neurology. Neurology 2016; 87:815-21
- MOJADIDI MK, ZAMAN MO, ELGENDY IY, et al. Cryptogenic stroke and patent foramen ovale. J Am Coll Cardiol 2018; 71:1035-43
- OVERELL JR, BONE I, LEES KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000; 55:1172-9
- PATEL PA, HALL A, AUGOUSTIDES JGT, et al. Dynamic shunting across a patent foramen ovale in adult cardiac surgery – Perioperative challenges and management. J Cardiothorac Vasc Anesth 2018; 32:542-9
- SAVER JL, CARROLL JD, THALER DE; et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med 2017; 377:1022-32
- SCHNEIDER B, ZIENKIEWICZ T, JANSEN V, et al. Diagnosis of patent foramen ovale by transesophageal echocardiography and correlation with autopsy findings. Am J Cardiol 1996; 77:1202-9
- SILVERSIDES CK, DORE A, POIRIER N, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Shunt lesions. Can J Cardiol 2010; 26:e70-e79
- SØNDERGAARD L, KASNER SE, RHODES JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med 2017; 377:1033-42
- STOUT KK, DANIELS CJ, VALENTE AM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease. J Am Coll Cardiol 2019; 73:e81-192
- SUKERNIK MR, GOSWAMI S, FRUMENTO RJ, et al. National survey regarding the management of an intraoperatively diagnosed patent foramen ovale during coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2005; 19:150-4
- SUKERNIK MR, METS B, BENNETT-GUERRERO E. Patent foramen ovale and its significance in the perioperative period. Anesth Analg 2001; 93:1137-46
- TORTI S. L’importance du formane ovale perméable en plongée, avec les recommendations 2007 de la Société Suisse de Médecine Subaquatique et Hyperbare (Importance of patent foramen ovale in diving with 2007 recommendations from the Swiss Underwater and Hyperbaric Medicine Society). Forum Med Suisse 2007; 7:975-7
- WARNES CA, WILLIAMS RG, BASHORE TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008; 118:2395-451
