Lowering cellular metabolism through cooling offers the organs a certain degree of protection and enables ischaemia time to be prolonged for a variable period depending on the temperature. Due to this reduction in requirements, it is possible to lower the perfusion flow rate, which offers undeniable benefits.
- Reduction of haematological trauma;
- Reduced collateral flow and pulmonary venous flow in the operating field;
- Option of discontinuing CPB for a short period or even remove some cannulas, which enables an improvement in the quality of surgical reconstruction in an immobile and blood-free operation field;
- Reduced myocardial rewarming following cold cardioplegia (4°C);
- Safety margin in the event of incidents requiring the CPB to be suddenly reduced or stopped.
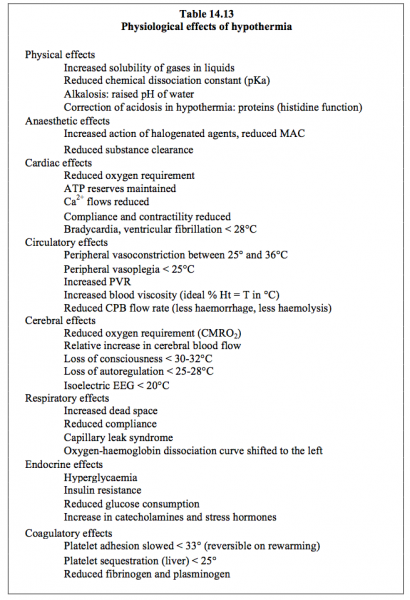
The significant haemodilution required to slow the increase in blood viscosity due to the cold results in severe cellular oedema in immature hearts and lungs – their compliance is reduced. Hypothermia, which may be minor (30-34°C), moderate (23-30°C) or deep (15-22°C), has repercussions on numerous systems (Table 14.13) (see Chapter 18 - Cerebral Effects of Hypothermia). While it offers safer conditions for correcting complex heart diseases, but it is equivalent to normothermia (≥ 34°C) for simple heart diseases [36].
Deep hypothermia
Cellular metabolism decreases exponentially with the temperature – it falls by 7% per degree C. At 18°C, the cerebral O2 requirement (CMRO2) is 40% of its value at normothermia [24]. The metabolic reduction coefficient per 10°C decrement (Q10) is a mean value of 2.5 in adults and 3.65 in neonates [25]. Besides reducing VO2, hypothermia preserves high-energy phosphates and impedes the entry of Ca2+ into cells, although it causes Ca2+ to be released from the sarcoplasmic reticulum. This increase in [Ca2+]i may result in stone heart, prompting post-CPB ventricular failure. The resulting vasoconstriction is most pronounced in the muscles of the limbs and slightly less pronounced in the kidneys and splanchnic system (see Chapter 7 - Hypothermia). Once the metabolism is reduced, it is possible to lower the CPB blood flow based on blood temperature, although consideration should be given to infants’ higher baseline metabolism compared to adults (8-9 mL O2/kg/min versus 4 mL O2/kg/min at 37°) and VO2 reduction related to anaesthesia [34].
- ≥ 34°C 2.5 L/min/m2
- 32° 2.2 L/min/m2
- 30° 2.0 L/min/m2
- 28° 1.8 L/min/m2
- 26° 1.6 L/min/m2
- 24° 1.4 L/min/m2
- 22° 1.2 L/min/m2
- 20° 1.0 L/min/m2
- 18° 0.7 L/min/m2
Flow compatibility with the body's requirements is continuously monitored by SvO2 and ScO2, supplemented by blood-gas analysis and measurements of lactate levels every 30 minutes.
The relationship between cerebral blood flow (CBF) and cerebral metabolism (normal CBF/CMRO2: 15/1) changes at low temperatures, with CBF becoming luxuriant (ratio of 60/1) [31]. In normocapnia, autoregulation of cerebral blood flow is maintained during moderate hypothermia (25-30°C) at mean arterial pressure levels of 50 mmHg. It is lost during deep hypothermia (< 23°C). In such instances, cerebral perfusion becomes pressure-dependent [12], although the CPB flow rate can be reduced as low as 10 mL/kg/min at 18°C before metabolic requirements are in deficit [20,21]. The lower temperature limit tolerated by the brain is probably 12°C, provided that hypothermia is homogeneous [20,22]. Below this value, the ions are able to diffuse according to their electrochemical gradients due to the inhibition of active membrane transfers (Na+/K+ and Na+/Ca2+ pumps), which causes progressive and irreversible intracellular oedema [30]. The most reliable clinical measurement of cerebral temperature is a tympanic or nasal probe (placed in contact with the ethmoidal sinuses).
Circulatory arrest
When operating on complex malformations such as hypoplastic left heart syndrome requiring a blood-free operating field, ablation of cannulas, and correction of the aortic arch, low temperatures are used (15-18°C), enabling work to be performed during circulatory arrest. By lowering the metabolism, it is possible to discontinue cerebral circulation for a period of time. However, there is always a risk of neurological sequelae, irrespective of the temperature and duration – only the probability of irreversible lesions is quantifiable. This is illustrated by Figure 14.26, which summarises so-called “safe” durations of complete arrest according to temperature [22,23]:
The relationship between cerebral blood flow (CBF) and cerebral metabolism (normal CBF/CMRO2: 15/1) changes at low temperatures, with CBF becoming luxuriant (ratio of 60/1) [31]. In normocapnia, autoregulation of cerebral blood flow is maintained during moderate hypothermia (25-30°C) at mean arterial pressure levels of 50 mmHg. It is lost during deep hypothermia (< 23°C). In such instances, cerebral perfusion becomes pressure-dependent [12], although the CPB flow rate can be reduced as low as 10 mL/kg/min at 18°C before metabolic requirements are in deficit [20,21]. The lower temperature limit tolerated by the brain is probably 12°C, provided that hypothermia is homogeneous [20,22]. Below this value, the ions are able to diffuse according to their electrochemical gradients due to the inhibition of active membrane transfers (Na+/K+ and Na+/Ca2+ pumps), which causes progressive and irreversible intracellular oedema [30]. The most reliable clinical measurement of cerebral temperature is a tympanic or nasal probe (placed in contact with the ethmoidal sinuses).
Circulatory arrest
When operating on complex malformations such as hypoplastic left heart syndrome requiring a blood-free operating field, ablation of cannulas, and correction of the aortic arch, low temperatures are used (15-18°C), enabling work to be performed during circulatory arrest. By lowering the metabolism, it is possible to discontinue cerebral circulation for a period of time. However, there is always a risk of neurological sequelae, irrespective of the temperature and duration – only the probability of irreversible lesions is quantifiable. This is illustrated by Figure 14.26, which summarises so-called “safe” durations of complete arrest according to temperature [22,23]:
- 3-5 min at 37°C;
- 12-15 min at 28°C;
- 35-40 min at 18°C.
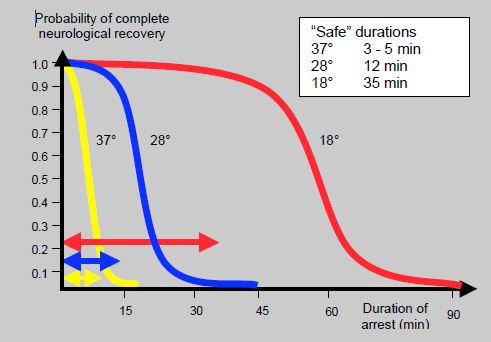
Figure 14.26: Nomogram showing estimated probability of full neurological recovery following total circulatory arrest at three different cerebral temperatures [20,23].
These durations are measured up to the point at which the curve bends, when the probability of neurological sequelae significantly increases. However, this is merely a probability – it is never zero and any prolongation of the duration increases the risk. The main operations during which deep hypothermia and circulatory arrest are normally applied are: total anomalous pulmonary venous return, Norwood, hemi-Fontan, arterial switch with VSD and interruption of the aortic arch [29].
Cerebral perfusion
A continuous low flow rate during deep hypothermia is a compromise aimed at maintaining the required supply for minimal cell function, preventing the accumulation of acid metabolites, and eliminating reperfusion lesions. Compared to total circulatory arrest, deep hypothermic low-flow ensures fewer immediate neurological complications (OR - 11.4) [26] and improved subsequent cognitive development [2,5]. However, many comparative studies show no significant difference between moderate hypothermic continuous low-flow and deep hypothermic total circulatory arrest [11,16]. It is difficult to determine the best strategy, since approaches differ between centres [34]. However, any protocol is effective if it is applied rigorously by a well-organised team that is fully familiar with it.
Metabolic requirements are theoretically met by a cerebral flow rate of 10-12 mL/kg/min at 15°C [8]. The brain can be selectively perfused by a cannula in the brachiocephalic trunk in order to protect it with continuous low flow during the circulatory arrest phase required for cardiac reconstruction. The right carotid artery is directly perfused and connexions with the external carotid system and the circle of Willis perfuse the left hemisphere [28]. Depending on the temperature, flow rates of 20-50 mL/kg/min are used at mean pressures of 20-40 mmHg. Since cerebral autoregulation is lost below 20°C, cerebral blood flow becomes linearly dependent on pressure at these temperatures. However, it drops suddenly to zero if the pressure falls to values of 11-15 mmHg [32]. It is therefore important that pressure is kept above these values. Moreover, jugular venous pressure must be zero otherwise it compromises the cerebral blood flow [13].
Technical aspects
Cooling by CPB is very gradual. To ensure homogeneity, it must be slow – ideally at least 20 minutes should be allowed to reach 20°C [3]. The level of neuromuscular blockade and the depth of anaesthesia must be sufficient to prevent shivering and minimise any peripheral oxygen requirement (VO2). During CPB, curarisation is likely to reduce overall VO2 by 10-30% [19]. Using vasodilator agents and pH-stat regulation helps ensure steady cooling and prevent the emergence of temperature gradients. Optimal haematocrit is 24%. A number of recommendations apply to temperature management during CPB [10].
- The temperature gradient between the heat exchanger water and the blood must never exceed 10°C and the water temperature must not exceed 38° or fall below 12°C.
- During cooling, the temperature gradient between the heat exchanger inlet and outlet must never exceed 10°C.
- When the temperature is < 30°C during rewarming, the temperature gradient between the heat exchanger inlet and outlet must never exceed 10°C.
- When the temperature is > 30°C during rewarming:
- The temperature gradient between the heat exchanger inlet and outlet must remain ≤ 4°C;
- The rewarming speed must remain ≤ 0.5°C/min.
- Blood temperature at the heat exchanger outlet must never exceed 37°C to prevent cerebral hyperthermia.
- The gradient between rectal/bladder temperature and oesophageal temperature must remain lower than 10°C – rectal or bladder T° is 2-4°C lower than brain temperature during rewarming.
In addition to hypothermia, a number of steps are taken to protect the brain in the event of circulatory arrest (see Cerebral Protection).
- External cooling with crushed ice, which is packed around the child's head and neck. The temperature of the operating theatre is lowered to 16°C until rewarming.
- Although the Trendelenburg position prevents air embolism if vessels are open or being manipulated, it increases venous pressure and may reduce effective cerebral perfusion pressure (Partery - Pvein) if it is too deep.
- Normoglycaemia – hypoglycaemia entails greater risk for young children. It is advised to adjust blood glucose levels to 6-10 mmol/L prior to arrest.
- Steroids: methylprednisolone reduces perifocal oedema due to its stabilising effect on the cell membranes [1]. Although it is unlikely to be effective for managing ischaemia, a single dose is safe, even at high dosage, and can therefore be justified for prophylaxis as it reduces the intensity of the inflammatory response [35]. The dose is 10-20 mg/kg administered 45 minutes prior to arrest.
- Mannitol: it reduces cerebral oedema and helps improve parenchymal perfusion – it can be used to reduce reperfusion lesions due to its ability to capture free radicals [37]. It is administered 20-30 minutes prior to arrest at a dose of 0.5 g/kg.
- Magnesium: as a sulphate or chloride, it has a powerful calcium channel-blocking effect and improves neurological recovery according to some studies [33]. The dose is 5-10 mmol 5 minutes prior to arrest.
- pH-stat regulation during the cooling and rewarming phases [4,9,18].
- Relatively high haematocrit should be maintained (> 24%) [16].
Barbiturates lower CMRO2 by 30% and improve focal lesion recovery, but not global cerebral ischaemia recovery [27]. However, embolic focal lesions are rarer than global ischaemic sequelae in young children. Moreover, barbiturates’ haemodynamic effects are prohibitive. Consequently, these agents are not used for cerebral protection in children.
There is no clear evidence to suggest that the various prophylactic methods mentioned here are able to significantly alter cerebral recovery. Only hypothermia and arrest brevity have a proven impact on long-term neurological outcomes.
Rewarming
During rewarming, the temperature gradient between the heat exchanger inlet and outlet must never exceed 10°C while the temperature is < 30°C. Once it is > 30°C, the temperature gradient must remain ≤ 4°C and the rewarming speed must remain ≤ 0.5°C/min. Moreover, blood temperature at the heat exchanger outlet must never exceed 37°C [10]. The artery-oesophagus temperature gradient must remain at 2-3°C [7]; However, the drawback of this gradual rewarming process is prolonged CPB duration [15].
If rewarming is performed rapidly, the brain becomes dangerously hyperthermic (38-39°) for several hours [6]. Indeed, since it is one of the most generously perfused organs, its temperature changes faster than body temperature. This phenomenon profoundly exacerbates the neurons’ susceptibility to ischaemia and increases the extent of focal lesions. It limits the efficacy of autoregulation and makes the cerebral blood flow more pressure-dependent. Neurological sequelae are also proportionate to the rewarming speed and to the fall in jugular venous saturation during rewarming [14,15]. A drop in cerebral O2 saturation (ScO2) indicates an imbalance between the O2 requirement and the cerebral blood flow. Cerebral hyperthermia is responsible for 50-75% of neuropsychological complications whose severity is directly linked to the rewarming speed [17].
There is no clear evidence to suggest that the various prophylactic methods mentioned here are able to significantly alter cerebral recovery. Only hypothermia and arrest brevity have a proven impact on long-term neurological outcomes.
Rewarming
During rewarming, the temperature gradient between the heat exchanger inlet and outlet must never exceed 10°C while the temperature is < 30°C. Once it is > 30°C, the temperature gradient must remain ≤ 4°C and the rewarming speed must remain ≤ 0.5°C/min. Moreover, blood temperature at the heat exchanger outlet must never exceed 37°C [10]. The artery-oesophagus temperature gradient must remain at 2-3°C [7]; However, the drawback of this gradual rewarming process is prolonged CPB duration [15].
If rewarming is performed rapidly, the brain becomes dangerously hyperthermic (38-39°) for several hours [6]. Indeed, since it is one of the most generously perfused organs, its temperature changes faster than body temperature. This phenomenon profoundly exacerbates the neurons’ susceptibility to ischaemia and increases the extent of focal lesions. It limits the efficacy of autoregulation and makes the cerebral blood flow more pressure-dependent. Neurological sequelae are also proportionate to the rewarming speed and to the fall in jugular venous saturation during rewarming [14,15]. A drop in cerebral O2 saturation (ScO2) indicates an imbalance between the O2 requirement and the cerebral blood flow. Cerebral hyperthermia is responsible for 50-75% of neuropsychological complications whose severity is directly linked to the rewarming speed [17].
| Hypothermia |
|
Hypothermia reduces tissue metabolic requirements (7%/°C reduction for the brain), enables the CPB flow rate to be reduced (1.8 L/min/m2 at 28°, 1.0 L/min/m2 at 20°), and allows it to be discontinued temporarily. Duration of circulatory arrest considered as safe:
- 3-5 minutes at 37°C - 15 minutes at 28°C - 40 minutes at 18°C On rewarming, a rebound effect causes cerebral hyperthermia, which is very harmful. Maximum rewarming speed: 1°/2-4 min; gradient between the heat exchanger and rectal T°: < 10°C; Blood T°: ≤ 37°C. Cerebral protection measures: - Continuous selective perfusion - Hypothermia at 18-20°C, steady and slow cooling and rewarming (1°/3-4 min), risk of cerebral hyperthermia on rewarming - Moderate hypothermia (28-30°C), continuous cerebral + splanchnic perfusion by right subclavian and femoral cannulation - Trendelenburg position - Normoglycaemia, mannitol (↓ cerebral oedema) - Deep anaesthesia (curarisation) - Unproven: Mg2+, thiopental, nimodipine, methylprednisolone |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- ALTMAN DI, YOUNG RSK, YAGEL SK. Effects of dexamethasone in hypoxic ischemic brain injury in the neonatal rat. Biol Neonate 1984:46:149
- BELLINGER DC, NEWBURGER JW, WYPIJ D, et al. Behavior at eight years in children with surgically corrected transposition: the Boston Circulatory Arrest Trial. Cardiol Young 2009; 19:86-9
- BELLINGER DC, WERNOWSKY G, RAPPAPORT LA, et al. Cognitive developpment of children following early repair of transposition of the great arteries using deep hypothermic circulatory arrest. Pediatrics 1991 ; 87:701-7
- BELLINGER DC, WYPIJ D, DU PLESSIS AJ, et al. Developmental and neurologic effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg 2001; 121:374-83
- BELLINGER DC, WYPIJ D, KUBAN KC, et al. Developmental and neurologic status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation 1999; 100: 526-32
- BISSONNETTE B, HOLTBY HM, PUA DAJ, et al. Cerebral hyperthermia in children after cardiopulmonary bypass. Anesthesiology 2000; 93:611-8
- BORGER MA, RAO V. Temperature management during cardiopulmonary bypass: Effect of rewarming rate on cognitive dysfunction. Semin Cardiothorac Vasc Anesth 2002; 6:17-20
- CROUGHWELL N, SMITH LR, QUILL T, et al. The effect of temperature on cerebral metabolism and blood flow in adults during cardiopulmonary bypass. J Thor Cardiovasc Surg 1992; 103:549-54
- DU PLESSIS AJ, JONAS RA, WYPIJ D. Perioperative effects of -stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg 1997; 114:991-1000
- ENGELMAN R, BAKER RA, LIKOSKY DS, et al. The Society of Thoracic Surgeons, the Society of Cardiovascilar Anesthesiologists, and the American Society of Extracorporeal Technology: clinical practice guidelines for cardiopulmonary bypass – Temperature management during cardioplmonary bypass. J Cardiothorac Vasc Anesth 2015; 29:1104-13
- GOLDBERG CS, BOVE EL; DEVANEY EJ, et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest; outcomes for infants with functional single ventricle. J Thorac Cardiovasc Surg 2007; 133:880-7
- GREELEY WJ, KERN FH, UNGERLEIDER RM, ET AL. The effect of hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral metabolism in neonates, infants and children. J Thorac Cardiovasc Surg 1991 ; 101 :783-94
- GREELEY WJ, UNGERLEIDER RM, SMITH LR, et al. The effects of deep hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral blood flow in infants and children. J Thorac Cardiovasc Surg 1989; 97(5):737-45
- GRIGORE AM, GROCOTT HP, MATHEW JP, et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg 2002; 94:4-10
- GRIGORE AM, MURRAY CF, RAMAKRISHNA H, et al. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass : does rewarming rate matter ? Anesth Analg 2009 ; 109 :1741-51
- HIRSCH JC, JACOBS ML, ANDROPOULOS D, et al. Protecting the infant brain during cardiac surgery: a systematic review. Ann Thorac Surg 2012; 94:1365-73
- HOGUE CW, PALIN CA, ARROWSMITH JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence- based appraisal of current practices. Anesth Analg 2006; 103:21-37
- HOOVER LR, DINAVAHI R, CHENG WP, et al. Jugular venous oxygenation during hypothermic cardiopulmonary bypass in patients at risk for abnormal cerebral autoregulation: influence of -stat versus pH-stat blood gas management. Anesth Analg 2009; 108:1389-93
- IRISH CL, MURKIN JM, CLELAND A, et al. Neuromuscular blocked significantly decreases systemic oxygen consumption during hypothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1991; 5:132-4
- KERN FH, GIESER WG, FARRELL DM. Extracorporeal circulation and circulatory assist devices in the pediatric patient. In: Lake CL. Pediatric cardiac anesthesia, 2nd edition. Norwalk : Appleton & Lange, 1993, pp 151-79
- KERN FH, UNGERLEIDER RM, QUILL TJ, et al. Cerebral blood flow response to changes in arterial carbon dioxide tension during hypothermic cardiopulmonary bypass in children. J Thorac Cardiovasc Surg 1991 ; 101 :618-22
- KERN FH, UNGERLEIDER RM, REVES JG, et al. The effect of altering pump flow rate on cerebral blood flow and cerebral metabolism in neonates, infants and children. Ann Thorac Surg 1993; 56:1366-72
- KIRKLIN JW, BARRAT-BOYES BG. Cardiac Surgery, 2nd edition, New-York: Churchill-Livingstone 1993, p 74
- MITCHENFELDER JD. The hypothermic brain. In: Anesthesia and the brain. New-York: Churchill-Livingstone, 1988, 23-34
- MITCHENFELDER JD, MILDE JH. The relationship among canine brain temperature, metabolism and function during hypothermia. Anesthesiology 1991; 75:130-6
- NEWBURGER JW, JONAS RA, WERNOVSKY G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 1993; 329:1057-64
- NUSSMEIER NA, ARLUNG C, SLOGOFF S. Neuropsychological dysfunction after cardio-pulmonary bypass: cerebral protectiono by a barbiturate. J Cardiothorac Vasc Anesth 1991; 5:584-9
- PIGULA FA, KHALIL PN, MAYER JE, et al. Repair of tetralogy of Fallot in neonates and young infants. Circulation 1999; 100(Suppl II):157-61
- POUARD P, MAURIAT P, LABORDE N, BOURDARIAS B. Cicrulation extracorporelle en chirurgie cardiaque pédiatrique chez le nouveau-né, le nourrisson et l’enfant. In: JANVIER G, LEHOT JJ, eds. Circulation extracorporelle: principes et pratique, 2ème édition. Paris: Arnette (Groupe Liaison SA) 2004, 481-506
- REBEYKA I. Hypothermia. In: Jonas RA, Elliott MJ. Cardiopulmonary bypass in neonates, infants and young children. Oxford: Butterworth, 1994, 54-66
- SWAIN JA, McDONALD TJ, ROBBINS RC, et al. Relationship of cerebral and myocardial intracellular pH to blood pH during hypothermia. Am J Physiol 1991 ; 260 :H1640-4
- TAYLOR RH, BURROWS FA, BISSONNETTE B. Cerebral pressure-flow velocity relationship during hypothermic cardiopulmonary bypass in neonates and infants. Anesth Analg 1992; 74:636-42
- WESTERMAIER T, HUNGERHUBER E, ZAUSINGER S, et al. Neuroprotective efficacy of intra-arterial and intravenous magnesium sulfate in a rat model of transient focal cerebral ischemia. Acta Neurochir 2003; 145:393-9
- WHITING D, YUKI K, DINARDO JA. Cardiopulmonary bypass in the pediatric population. Best Pract Res Clin Anaesthesiol 2015; 29:241-56
- WHITLOCK RP, CHAN S, DEVEREAUX PJ, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass : a meta-analysis of randomized trials. Eur Heart J 2008 ; 29 :2592-600
- XIONG Y, SUN Y, JI B, et al. Systematic review and meta-analysis of benefits and risks between normothermia and hypothermia during cardiopulmonary bypass in pediatric cardiac surgery. Paediatr Anaesth 2015; 25:135-42
- YOSIUMOTO T, SAKAMOTO T, WATANABE T, et al. Experimental cerebral infarction, part 3: Protective effect of mannitol in thalamic infarction in dogs. Stroke 1978; 9:217-22
