Activation of coagulation
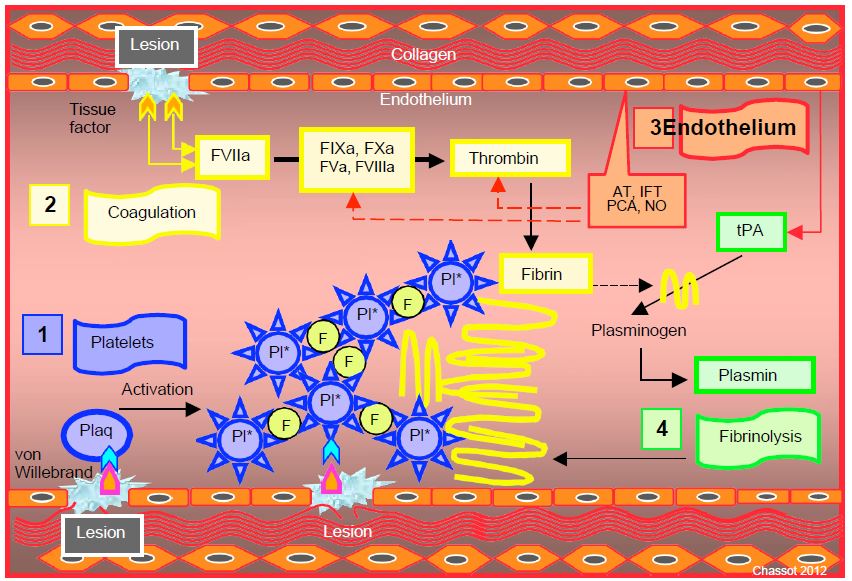
Coagulation is a local phenomenon, usually triggered by tissue damage that breaks the endothelial barrier (for more details see Chapter 8 Coagulation & Haemostasis). Four physiological processes are involved (Figure 7.20) [1,16].
- Platelet plug. Endothelial injury allows contact between tissue factors (von Willebrand, GPIb/V/IX) and circulating platelets; the latter move from pause to the activated phase. Activated platelets crowd each other by binding through their GPIIb/IIIa receptor attachments with fibrinogen. The activated platelets and the endothelial injury provide a surface of negatively charged phospholipids which is the platform on which the coagulation cascade is built.
- Coagulation cascade. Exposure of tissue factor (TF) within the endothelial damage activates circulating Factor VII to F VIIa, which triggers the coagulation chain (extrinsic pathway) leading to the conversion of prothrombin (F II) to thrombin (F IIa), which in turn potentiates platelet activation and coagulation by feedback in an amplifying loop (stimulation of Factors Va and VIIIa, which in turn stimulate Factors XIa and Xa). Thrombin converts circulating fibrinogen into fibrin, which stabilises the platelet plug and transforms it into a solid thrombus through the action of activated factor XIII (F XIIIa), which creates bridges between the fibrin molecules.
- Normal endothelial cells act as regulators to prevent the spread of thrombi beyond the injured area. They release a series of factors that inhibit platelets and the coagulation cascade (antithrombin, NO, tissue factor pathway inhibitor) which interrupt the coagulation pathway and contain it at the site of injury.
- Fibrinolysis. The endothelium also releases tissue plasminogen activator, which binds to fibrin and converts plasminogen to plasmin, which lyses the fibrin and breaks it into fragments with no coagulant activity (D-dimers).
Independent of any tissue damage, ECC directly triggers the formation of thrombin and fibrin (intrinsic pathway). Five minutes after the start of the procedure, their level is already increased by 20 times, although these substances are normally only found in the wound and not in the systemic circulation [5]. Several phenomena are involved [12,25].
Figure 7.20: Activation of the coagulatory cascade in a tissue injury such as the surgical wound. Four processes are involved. 1: Platelet plug (blue). Von Willebrand factor is brought into contact with the platelets by the endothelial injury; the platelets move from the resting (Plaq) to the activated (Pl*) phase. Activated platelets cluster together by binding to fibrinogen (F) via their GPIIb/IIIa receptor attachments. 2: Coagulation cascade (yellow). Exposure of tissue factor in the lesion activates circulating Factor VII to FVIIa, which sets the coagulation chain in motion, leading to the transformation of prothrombin into thrombin, which potentiates coagulation by feedback in an amplifying loop (stimulation of factors Va and VIIIa which in turn stimulate factors XIa and Xa). Thrombin converts the circulating fibrinogen into fibrin, which stabilises the platelet plug (yellow filaments) and transforms it into a solid thrombus thanks to the action of activated factor XIII (F XIIIa), which creates bridges between the fibrin molecules. 3: Normal endothelial cells act as regulators (in red) to prevent the propagation of coagulation beyond the injured area. They release a series of inhibitory factors (antithrombin AT, active protein C PCA, tissue factor pathway inhibitor IFT, NO) that interrupt the coagulation pathway. 4: Fibrinolysis (green). The endothelium also releases tissue plasminogen activator (tPA) which binds to fibrin and converts plasminogen to plasmin, which lyses fibrin [25].
- The contact system. On contact with negatively charged foreign surfaces such as glass or plastics, factor XII (Hageman) cleaves to (activated) XIIa, which converts prekallikrein to kallikrein, factor XI to XIa and kininogens to bradykinin; the latter increases 10-fold. Activation of FXIa leads to the active formation of thrombin via the intrinsic coagulation pathway. Factor XIIa also activates the complement pathway and promotes the conversion of plasminogen to plasmin, leading to fibrinolysis. However, the balance is in favour of excess thrombin and the procoagulant effect; this situation persists until the 5th postoperative day [2].
- The extrinsic pathway. Tissue factor (TF) expression and factor VIIa concentration are abnormally high in ECC.
- Fibrinolysis. Circulating plasmin levels increase 10-50-fold during ECC; since the rates of fibrin formation and degradation are equivalent, this leads to increased fibrinogen consumption without clot formation [6].
- Platelets. Platelets are stimulated by contact with foreign surfaces, by heparin and by excess circulating thrombin; they adhere to surfaces, form clusters and secrete vasoconstrictor thromboxane A2. Their number and aggregability decrease by 30-50% during ECC, partly due to mechanical damage in pumps and circuits [24]. Their function is reduced in hypothermia (< 30°C), but this dysfunction is reversible on rewarming; however, it does not appear on the result of aggregability tests that are performed on blood warmed to 37°C. Plasmin dissociates the GP Ib receptor, which partially activates the platelet but makes it less sensitive to agonists [9].
- The inflammatory response. Leukocytes are activated by foreign surfaces and secrete tissue factor (TF), which contributes to the development of the coagulation cascade and the production of thrombin. Activated leukocytes infiltrate between endothelial cells and produce free radicals, superoxides and lysosomal enzymes; this causes endothelial damage, increased capillary leakage, extracellular fluid accumulation and inflammatory syndrome. Foreign surfaces also stimulate the alternative complement pathway (the classical pathway is already activated by F XIIa); C3a and C5a factors bind to circulating leukocytes and contribute to their activation.
- Hemodilution. The levels of all factors are lowered by 20-30% through dilution by priming volume of the ECC. Colloids lower factor VIII and von Willebrand levels and inhibit platelet adhesion [10,24].
- By the end of ECC, fibrinogen levels have fallen by 30-40%; 30% of antithrombin III is consumed, which tends to progressively increase "resistance" to heparin. Factors II, VII, IX and X are decreased by almost 50% after ECC [11].
- Retransfused blood. If not treated by a CellSaver™ system, blood sucked from the pericardium or mediastinum contains tissue factor, thrombin-antithrombin complexes, plasminogen activators and inflammatory triggers. It contributes massively to coagulation alterations.
- Hypothermia and acidosis. Coagulopathy sets in at 35°C, and the coagulation cascade is completely inactivated at 16°C. Acidosis worsens the situation and slows down the activity of pH-sensitive factors such as factor VIIa.
- Damage to platelets and coagulation factors (protein denaturation) is directly related to the duration of ECC, depth of hypothermia (≤ 25°), suctions, and contact with air (venous reservoir, suctions).
Tissue factor (TF) and extrinsic pathway expression is primarily related to surgical trauma, activation by suctions, and the inflammatory response. Activated leukocytes infiltrate between endothelial cells and produce free radicals, superoxides and lysosomal enzymes, resulting in endothelial damage, increased capillary permeability, extracellular fluid accumulation and systemic inflammatory syndrome (see Inflammatory syndrome). Damage to platelets and coagulation factors (protein denaturation) is directly related to the duration of ECC, depth of hypothermia (≤ 25°), suctioning, and contact with air (venous reservoir, suctioned).
Activation of coagulation can be reduced by various means, but, apart from anticoagulation, their effectiveness is highly variable.
- Complete anticoagulation with non-fractionated heparin (UFH); thrombin activity is blocked when the ACT is > 480 seconds, provided that sufficient anti-thrombin is present (see Heparins). The loading dose of heparin to achieve adequate anticoagulation is 300-400 IU/kg. ACT is checked 3-5 minutes later. Additional doses are titrated according to the patient's individual response to heparin.
- In case of heparin resistance, supplementation with anti-thrombin (AT III), as its level drops by 40% in ECC due to hemodilution and consumption by heparin is advised. The nadir of AT III concentration is reached on the 3rd postoperative day [8]. Administration in the form of AT III concentrate (500-1,000 IU for an adult) or fresh frozen plasma (see below) [31].
- Patients treated with anticoagulants or antiplatelets, receive the usual dose of heparin during ECC or OPCAB as the usual routine (ACT sought: > 450 sec and > 300 sec respectively), since incomplete inhibition of thrombin may lead to secondary platelet activation [4].
- Antifibrinolytics; tranexamic acid and amino caproic acid bind to plasminogen lysine and block plasmin activation and hence fibrinolysis. Aprotinin is a non-specific protease inhibitor, which directly blocks plasmin (see Anti-fibrinolytics).
- Thromboplegia; ECC and heparin activate platelets, which release their granules (ADP, thrombexane), form aggregates and adhere to surfaces; 30-50% of them are no longer functional postoperatively and no longer respond to ADP or collagen [24]. Their momentary blockage by a P2Y12 receptor (ADP receptor) antagonist such as cangrelor in perfusion (half-life: 9 minutes) protects them from stimulation and preserves their functionality for the postoperative period [18]. This promising therapy is still in trial phase.
- Modifications related to ECC technology [12].
- Restriction of suctioned blood; recovered blood is in contact with air and contains activators of coagulation (TF, thrombin), fibrinolysis (plasmin) and inflammation (interleukins, TNF, C3a, C5a). Suctioned blood is the main source of haemolysis, thrombocytopenia, coagulopathy and stimulation of the inflammatory syndrome [25]. Disruption of the coagulatory system is markedly reduced when suctioned blood is not recycled or is treated by CellSaver™ system, but this procedure unfortunately removes platelets, proteins and coagulation factors [30].
- Restriction of circuit size; miniaturisation of circuits and removal of the cardiotomy reservoir minimises contact of blood with foreign surfaces and suppresses contact with air, thereby inhibiting the release of coagulation activators and inflammatory triggers.
- Biocompatibility of circuits; preheparinised circuits and circuits impregnated with particular polymers inhibit the complement cascade, platelet aggregability and leukocyte activation. However, the clinical effect is small and limited to a decrease in postoperative AF and ICU stay [20]. The reduction in transfusions is unpredictable.
- Ultrafiltration; continuous filtration at the end of ECC after weaning off bypass (MUF, modified ultrafiltration) reduces hemodilution and removes many cytokines and inflammatory triggers.
- Beating heart surgery without ECC; the absence of ECC does not eliminate coagulo-inflammatory activation, but reduces it; platelet dysfunction is reduced [28].
Anticoagulation and coagulopathy related to ECC should be antagonised and treated respectively to limit bleeding risks. The antagonist of heparin is protamine, administered at a ratio of 1 mg protamine to 1 mg heparin (see Protamine). Excess protamine can inhibit the coagulation cascade and platelet activity. Protamine is injected as soon as the patient is decanulated. It has several side effects:
- Systemic vasodilation and arterial hypotension;
- Pulmonary vasoconstriction;
- Antigen-antibody reaction;
- Anaphylactoid reaction (anaphylactic shock).
| ECC coagulopathy |
|
ECC coagulopathy is related to 3 phenomena: hemodilution, activation and consumption. ECC rapidly triggers thrombin and fibrin formation, independent of any tissue injury. Five systems are activated by contact with foreign surfaces:
- Activated F XII triggers the intrinsic pathway
- Tissue factor and factor VIIa are increased
- Platelets are stimulated
- Activation of fibrinolysis destroys the fibrin formed but consumes fibrinogen
- Activation of leukocytes and complement triggers a massive systemic inflammatory response
systemic inflammatory response
Several means are used to counteract this stimulation:
- Complete anticoagulation (heparin 300-400 IU/kg)
- Antifibrinolytics
- Aspiration restriction, CellSaver™
- Miniaturisation of circuits, biocompatible circuits
- Ultrafiltration
- Beating heart surgery
|
Hemodilution
The mixing of blood with the priming fluid (1.0 - 1.5 L of hydroelectrolyte solution) is responsible for a major hemodilution that suddenly lowers the hematocrit to around 25% and decreases the colloid-osmotic pressure by 40% [15]. It is the main cause of pressure drop at the beginning of ECC. The pressure then rises again because hypothermia causes a stimulation of peripheral arterial resistance (PAR) and because viscosity increases as the blood temperature drops. The fall in osmotic pressure exacerbates extracellular fluid leakage into the interstitial space of the lungs, heart, liver, kidneys, abdominal viscera and muscles.
Hemodilution is beneficial in several ways (see Priming fluid).
- It improves microcirculation by lowering blood viscosity, which is crucial in hypothermia; viscosity remains stable when Ht in percent is the same as temperature in degrees C°.
- It tends to reduce the need for allologous blood and the complications associated with transfusion.
- It is well tolerated as tissue O2 consumption is reduced with cold.
In normothermic ECC, a Ht of 18% is just enough to meet the oxygen requirements of a sedated and curarised patient [19]. When Hb is <70 g/L, cerebral blood flow increases by 45% and renal plasma flow rises in the cortical area, but coronary reserve decreases by 50% and splanchnic perfusion is borderline ischaemic [22,26].
Hemodilution is only beneficial within certain limits. Ht below 22%, for example, is an independent predictor of postoperative morbidity and mortality [14]. Ht has a particular impact on brain function and renal function. Neurocognitive impairment becomes more important at a minimum Ht of 15-17% [7]; only Ht > 28% ensures normal postoperative neurological status [29]. In children, neurological score and psychomotor development are better at high Ht in ECC (28%) than at low Ht (21%) [17]. On the other hand, renal function worsens linearly with decreasing haemoglobin when hematocrit is < 30% [27]. A Ht of 25-28% during ECC is therefore the safe lower limit to ensure organ recovery.
Haemolysis
Contact with foreign surfaces of various kinds causes a series of haematological injuries of varying severity, which are generally directly proportional to the duration of ECC. In addition, the pumps cause mechanical damage to the figurative elements, which depends on their degree of occlusivity and their speed of rotation. Finally, suctioning in the operating field is responsible for some of the inflammatory and erythrocytic damage, which becomes serious the more powerful and prolonged the suctioning and the greater the bleeding. The resulting haemolysis is clearly visible in the urine, which turns brown. In this case, it is necessary to maintain a satisfactory urine flow and to alkalinise the urine with Na+ bicarbonate (50-100 mmol i.v.) to slow down the crystallisation of free Hb in the tubules [13].
Another cause of haemolysis is the presence of cold agglutinins. This is an autoimmune disease characterised by the presence of antibodies that cause agglutination of erythrocytes below a certain temperature threshold.
Cold agglutinins
Cold agglutinins are IgM antibodies directed against Anti-I antigens present on the membrane of red blood cells. They cause agglutination of red blood cells at low temperatures. On heating, these aggregates cause microvascular thrombi and are hemolyzed, releasing a large amount of free haemoglobin. This condition is an idiopathic disease, or the sequelae of an infectious or lymphoproliferative process. Its incidence is less than 1% of cardiac surgery patients. The condition manifests as peripheral thrombosis and haemolysis (see Chapter 21 Coagulopathies).
Cold agglutinins are detected by both direct (presence of complement on the patient's RBCs) and indirect (presence of serum antibodies) Coomb's test. They exist in all individuals, but normally only react at 0-4°C. Their clinical significance lies in their serum level and the temperature value at which they are activated. The values considered safe for ECC are a concentration of less than 1:32 at 4°C, with no detectable agglutination at 28°C or above. The likelihood of intraoperative complications becomes significant for levels above 1:512 at 4°C, or below this value if the activation temperature is above 25°C [21].
In the operating room, a number of precautions are set up [3].
- Surgery in normothermia (ECC > 34°C) or beating heart;
- Warming of the operating room and infusions;
- Warm (> 34°C) crystalloid or blood cardioplegia;
- Warming of blood bags in case of transfusion;
- In case of crisis with haemolysis:
- Warm to 37°C;
- Improve peripheral perfusion with a vasodilator (nitroprusside);
- Alkalinate urine (50-100 mmol Na+ bicarbonate);
- Methylprednisolone (500 mg): effectiveness discussed;
- Reduction of circulating levels by preoperative plasmapheresis if necessary.
- Seizures manifest as haemolysis and peripheral myocardial, hepatic and renal vascular occlusions.
| Haematological aspects |
|
The priming volume of the ECC causes hemodilution (Ht 25-28%), which is necessary to slow the increase in blood viscosity at low temperatures. In hypothermia, viscosity remains stable when the Ht in % is the same as the temperature in degrees C°. When Ht is < 25%, postoperative neurological status and renal function are impaired. A Ht of 25-28% is the safe lower limit to ensure normal organ function.
ECC causes haemolysis, usually sub-clinical. In hypothermia, this can become massive in the presence of cold haemagglutinins (demonstrated by a Coombs test).
ECC rapidly triggers the formation of thrombin and fibrin, independent of any tissue injury. Four systems are activated by contact with foreign surfaces:
- Activated F XII triggers the intrinsic pathway
- Platelets are stimulated
- Fibrinolysis destroys the fibrin formed but consumes fibrinogen
- Activation of leukocytes and complement triggers a massive systemic inflammatory response
|
CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- ACHNECK HE, SILESHI B, PARIKH A, et al. Pathophysiology of bleeding and clotting in the cardiac surgery patient. Circulation 2010; 122:2068-77
- AVIDAN MS, LEVY JH, SCHOLZ J, et al. A phase III, double-blind, placebo-controlled, multicenter study on the efficacy of recombinant human antithrombin in heparin-resistant patients scheduled to undergo cardiac surgery requiring cardiopulmonary bypass. Anesthesiology 2005; 102: 276-84
- BRACKEN CA, GURKOWSKI MA, NAPLES JJ, et al. Cardiopulmonary bypass in two patients with previously undetected cold agglutinins. Case Conference. J Cardiothorac Vasc Anesth 1993; 7:743-9
- BROWN C, JOSHI B, FARADAY N, et al. Emergency cardiac surgery in patients with acute coronary syndromes: a review of the evidence and perioperative implications of medical and mechanical therapeutics. Anesth Analg 2011; 112: 277-99
- CHANDLER WL, VELAN T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003; 101:4355-62
- CHANDLER WL, VELAN T. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coag Fibrinolysis 2004; 15:583-91
- DE FOE GR, ROSS CS, OLMSTEAD EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Ann Thorac Surg 2001; 71:769-76
- DIETRICH W, BUSLEY R, SPANNAGL M, et al. The influence of antithrombin substitution on heparin sensitivity and activation of hemostasis during coronary artery bypass surgery: a dose-finding study. Anesth Analg 2013; 116:1223-30
- DUNNING J, VERSTEEGH M, FABBRI A, et al. Guidelines on antiplatelet and anticoagulation management in cardiac surgery. Eur J Cardiothorac Surg 2008; 34:73-92
- FRANZ A, BRAUNLICH P, et al. The effects of hydroxyethyl starches of varying molecular weights on platelet function. Anesth Analg 2001; 92: 1402-7
- GHADIMI K, LEVY JH, WELSBY IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg 2016; 122:1287-300
- GRONCHI F, RANUCCI M. Perioperative coagulation in cardiovascular surgery. In: MARCUCCI C, SCHOETKER P, editors. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer Verlag, 2014, 243-66
- HAASE M, HAASE-FIELITZ A, BELLOMO R, et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med 2009; 37:39-47
- HABIB RH, ZACHARIAS A, SCHWANN TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg 2003; 125:1438-50
- HALL TS. The pathophysiology of cardiopulmonary bypass: The risks and benefits of hemodilution. Chest 1995: 100:88-94
- INNERHOFER P, KIENAST J. Principles of perioperative coagulopathy. Best Pract Res Clin Anaesthesiol 2010; 24:1-14
- JONAS RA, WYPIJ D, ROTH SJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 2003; 126:1765-74
- KRAJEWSKI S, KURZ J, NEUMANN B, et al. Short-acting P2Y12 blockade to reduce platelet dysfunction and coagulopathy during experimental extracorporeal circulation and hypothermia. Br J Anaesth 2012; 108:912-21
- LIAM BL, PLOCHL W, COOK DJ, et al. Hemodilution and whole body balance during normothermic cardiopulmonary bypass. J Cardiothorac vasc Surg 1998; 115:1203-8
- MANGOUSH O, PURKAYASTHA S, HAJ-YAHIA S, et al. Heparin-bonded circuits versus nonheparin-bonded circuits: an evaluation of their effect on clinical outcomes. Eur J Cardiothorac Surg 2007; 31:1058-69
- MONGERO LB, BECK JR (eds). On bypass. Advanced perfusion techniques. Policy and procedure guidelines (CP36): Cold agglutinins. Totowa (NJ, USA): Humana Press 2010, 426-8
- OHRI SK, BOWLES CW, MATHIE RT, et al. Effect of cardiopulmonary bypass perfusion protocols on gut tissue oxygenation and blood flow. Ann Thorac Surg 1997; 64:163-8
- RANUCCI M, BALDUINI A, DITTA A ; et al. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann Thorac Surg 2009; 87: 1311-9
- ROZENTAL T, SHORE-LESSERSON L. Pharmacologic management of coagulopathy in cardiac surgery: An update. J Cardiothorac Vasc Anesth 2012; 26:660-79
- SNIECINSKI RM, CHANDLER WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113:1319-33
- SUNGURTEKIN H, COOK DJ, ORSZULAK TA, et al. Cerebral response to hemodilution during hypothermic cardiopulmonary bypass in adults. Anesth Analg 1999; 89:1078-83
- SWAMINATHAN M, PHILIPS-BUTE BG, CONLON PJ, et al. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg 2003; 76:784-91
- UNTCH BR, JESKE WP, SCHWARTZ J, et al. Inflammatory and hemostatic activation in patients undergoing off-pump coronary artery bypass grafting. Clin Appl Thromb Hemost 2008; 14-141-8
- VAN WERMESKERKEN GK, LARDENOYE JWH, HILL SE, et al. Intraoperative physiologic variables and outcome in cardiac surgery. Part II. Neurologic outcome. Ann Thorac Surg 2000; 69:1077-83
- WANG G, BAINBRIDGE D, MARTIN J, et al. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg 2009; 109:320-30
- WILLIAMS MR, D'AMBRA AB, BECK JR, et al. A randomized trial of antithrombin concentrate for treatment of heparin resistance. Ann Thorac Surg 2000; 70:873-7