Several malformations of the left ventricular ejection area may occur. These are frequently observed in adults and are described in the chapters on valvular heart diseases (see Chapter 11, Aortic stenosis) and the thoracic aorta (Chapter 18 Ascending aorta). Congenital stenosis of the left ejection pathway may be located at three different sites:
- Subaortic in the LV outflow tract;
- Valvular, in the strict sense;
- Supravalvular at the root of the ascending aorta.
Obstructed outflow tract
Subaortic stenosis may be fixed or dynamic. Fixed stenosis involves a roughly circular membrane surrounding the outflow tract from the interventricular septum to the anterior leaflet of the mitral valve, and causes the equivalent of aortic stenosis (Video and Figure 15.48).
Video: Long-axis view of the left ventricular outflow tract with the presence of a sub-aortic membrane appearing as a fibrous spur on the septal wall of the LVOT.

Figure 15.48: Long-axis images of subaortic stenosis. The arrows indicate the membrane inserted on the septum and on the mitral valve (anterior leaflet).
The valve, which is situated distally to the membrane, is hypoplastic and incompetent in > 50% of cases, since the stenosis reduces the flow from birth and the post-stenotic jet causes trauma to the cusps [14]. This phenomenon requires surgery to be performed early in the course of the disease to protect the aortic valve, i.e. as soon as the mean gradient is > 30 mmHg (maximal gradient > 50 mmHg) [11,12,14]. It is commonly associated with coarctation of the aorta or a VSD. Subobstruction of the LVOT is a common complication of AV canal defect correction. It is linked to a gooseneck deformity of the outflow tract in the peculiar anatomy of this malformation (see AV canal defect) [2].
Stenosis may also be dynamic and functions in the same way as hypertrophic obstructive cardiomyopathy (HOCM) (see Chapter 11 Dynamic subaortic stenosis). This is characterised by velocity > 2.5 m/s (norm: 1.0-1.5 m/s) and a maximum gradient > 25 mmHg in the LVOT, and is exacerbated by beta sympathetic stimulation, hypovolaemia, and a reduction in peripheral resistance. Management comprises a β–blocker, hypervolaemia, and an α–stimulant. Surgical treatment involves resection of the membrane or a myectomy to widen the outflow tract. During surgery, there is a risk of creating a VSD through excessive resection, or a persistent gradient through insufficient resection. Perioperative TEE is very important for diagnosing an iatrogenic VSD or an excessive residual gradient. Immediate surgical revision is indicated in 12-25% of cases based on this examination [7,12].
Supravalvular stenosis
Aortic root narrowing presents as a diaphragm or hourglass deformity. It is located at the sinotubular junction or beyond the sinuses of Valsalva. Consequently, the coronary arteries are upstream of the stenosis and subjected to conditions of systolic hypertension. This situation causes these vessels to dilate, speeds up their atheromatous degeneration, and requires surgery to be performed early in the course of the disease [12,14]. Surgery consists of reconstructing the aortic root with reimplantation of the coronary arteries. This is indicated if the mean pressure gradient is ≥ 50 mmHg or the patient is symptomatic [10].
Bicuspid aortic valve
A bicuspid aortic valve is the most common adult congenital malformation, observed in 1-2% of patients [3]. It is often associated with coarctation of the aorta and more rarely with a perimembranous VSD. Aortic root anomalies linked to it may result in aortic dilation, an aneurysm, or a dissection [1]. A bicuspid aortic valve can be identified by the presence of two cusps rather than three. It is described as a "true" bicuspid aortic valve (type 0) if these two cusps are roughly equal in size with two commissural points and an ellipsoidal opening (Video and Figure 15.49) [9].
Video: Congenital bicuspid aortic valve; the leaflets are in antero-posterior position.

Figure 15.49: Bicuspid aortic valve A: there are only two commissures and the opening (white arrow) is ellipsoidal. B: bicuspid aortic valve in connection with Marfan syndrome; the valve opening is ellipsoidal, the borders of the two cusps do not reach the walls of the aorta during systole.
A bicuspid aortic valve is described as "false" (type 1 and 2) if there are three commissural points and two cusps have secondarily fused leaving a trace of this fusion in the form of a raphe. The opening is banana-shaped (Video). The current classification is based on the number of raphes (see Chapter 11 Bicuspidie aortique) [9].
Video: Calcified bicuspid aortic valve; the right and left coronary cusps are fused together, but there are still 3 commissures.
The bicuspid aortic valve is delicate and its cusps are subjected to more intense stress than a normal valve. When the valve is open, its extremities cannot reach the wall of the sinuses of Valsalva and vibrate during systole, causing a systolic murmur. When it undergoes fibrosis and calcification, it becomes stenotic, generally around the age of 40-50 years (Figure 15.50).
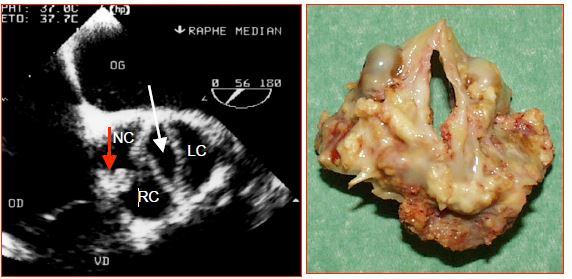
Figure 15.50: Bicuspid aortic valve A: "False" bicuspid aortic valve – there are three commissures (labelled 1,2 and 3), but the opening (white arrow) is ellipsoidal since the non-coronary (NC) and right coronary (RC) cusps are fused at the site of a calcified raphe (red vertical arrow) and only form one cusp. B: Anatomopathological specimen from the case presented in A. LC: left coronary cusp.
The valve can also become insufficient by dilation of the annulus, prolapse, or perforation of a cusp. This generally occurs at an earlier age than stenosis. It can usually be ascribed to aortic disease with variable degrees of stenosis and insufficiency (Video). The more eccentric the opening, the faster the progression. Auscultation of the aortic stenosis reveals a 5/6 ejection murmur with a thrill, a paradoxical split second heart sound and an opening click audible at the apex (for more details, see Chapter 11, Bicuspid aortic valve).
Video: Long-axis view of a bicuspid aortic valve; the colour flow shows a minor regurgitation and a severe stenosis.
Surgical options
In cases of a stenotic bicuspid aortic valve, surgery is indicated if the mean gradient is ≥ 40 mmHg, area ≤ 0.6 cm2/m2, the patient is symptomatic, or the LV is dilating [10,14]. In the event of aortic insufficiency (AI), surgery is indicated based on AI severity and LV dilation. In children and young adults, implantation of a prosthetic valve is avoided as mechanical prostheses require lifelong anticoagulation and biological prostheses only last 10 to 15 years (see Chapter 11 - Prosthetic valves). If the valve is flexible, non-calcified and free of insufficiency, the obstacle may be removed and ventricular remodelling prevented by means of percutaneous balloon valvotomy. However, there is a risk of creating residual insufficiency. The long-term success rate is equivalent to that of a prosthesis [8].
If a valvotomy is inappropriate given the specific circumstances, aortic valve repair (see Chapter 11 Other valve repairs) or a Ross procedure may be performed. This entails transposing the pulmonary valve to the aortic position (with reimplantation of the coronary arteries), and implanting a homograft in the pulmonary position (Figure 15.51; see Figure 11.146) [13].
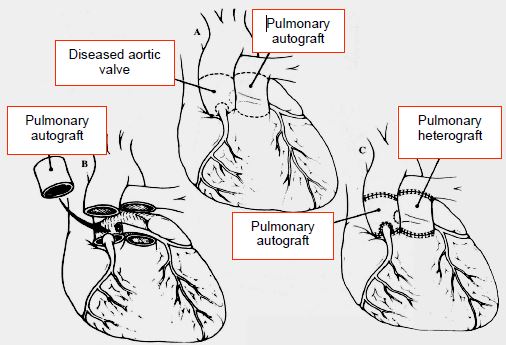
Figure 15.51: Ross procedure. This entails resecting the diseased aortic valve, implanting the patient's pulmonary valve in this position, and replacing the pulmonary valve with a valved conduit between the RV and PA.
This technique avoids anticoagulation and enables valve growth. The reason for this switch is that the rate of wear for homografts is high in the aortic position (30% at 10 years). Moreover, an incompetence in the pulmonary artery root is easier to manage since the right heart can accommodate major variations in volume; on the other hand, stenosis can be dilated by catheterisation without any risk of systemic embolism. The same wear-related lesions in the aortic position would require the implantation of a prosthesis. The Ross procedure is contraindicated in the event of pulmonary insufficiency, a bicuspid pulmonary valve, or major ventricular dysfunction. The surgical mortality rate for the Ross procedure is 3% and long-term mortality is < 1%/year. While the deterioration rate for the aortic autograft is 1%/year, it is higher for the homograft in the pulmonary artery root (2-3%/year). The neoaortic valve becomes insufficient in 30% of patients [4]. The reoperation rate above the age of 12 years is 10% for the aortic valve and 20-30% for the pulmonary homograft [13]. In patients aged over 40 years, the valve is replaced by a prosthesis, which ideally should be mechanical. Biological prostheses are used only in patients who cannot be anticoagulated or who are aged over 65 years. TAVI-type transcatheter implantation is not appropriate for a bicuspid aortic valve, but may be used for non-invasive treatment of stenosis or pulmonary homograft incompetence [5,6].
Anaesthesia
The requirements applicable to anaesthesia are the same as those for aortic stenosis (see Chapter 11 Aortic Stenosis, Anaesthesia).
Subaortic stenosis may be fixed or dynamic. Fixed stenosis involves a roughly circular membrane surrounding the outflow tract from the interventricular septum to the anterior leaflet of the mitral valve, and causes the equivalent of aortic stenosis (Video and Figure 15.48).
Video: Long-axis view of the left ventricular outflow tract with the presence of a sub-aortic membrane appearing as a fibrous spur on the septal wall of the LVOT.

Figure 15.48: Long-axis images of subaortic stenosis. The arrows indicate the membrane inserted on the septum and on the mitral valve (anterior leaflet).
The valve, which is situated distally to the membrane, is hypoplastic and incompetent in > 50% of cases, since the stenosis reduces the flow from birth and the post-stenotic jet causes trauma to the cusps [14]. This phenomenon requires surgery to be performed early in the course of the disease to protect the aortic valve, i.e. as soon as the mean gradient is > 30 mmHg (maximal gradient > 50 mmHg) [11,12,14]. It is commonly associated with coarctation of the aorta or a VSD. Subobstruction of the LVOT is a common complication of AV canal defect correction. It is linked to a gooseneck deformity of the outflow tract in the peculiar anatomy of this malformation (see AV canal defect) [2].
Stenosis may also be dynamic and functions in the same way as hypertrophic obstructive cardiomyopathy (HOCM) (see Chapter 11 Dynamic subaortic stenosis). This is characterised by velocity > 2.5 m/s (norm: 1.0-1.5 m/s) and a maximum gradient > 25 mmHg in the LVOT, and is exacerbated by beta sympathetic stimulation, hypovolaemia, and a reduction in peripheral resistance. Management comprises a β–blocker, hypervolaemia, and an α–stimulant. Surgical treatment involves resection of the membrane or a myectomy to widen the outflow tract. During surgery, there is a risk of creating a VSD through excessive resection, or a persistent gradient through insufficient resection. Perioperative TEE is very important for diagnosing an iatrogenic VSD or an excessive residual gradient. Immediate surgical revision is indicated in 12-25% of cases based on this examination [7,12].
Supravalvular stenosis
Aortic root narrowing presents as a diaphragm or hourglass deformity. It is located at the sinotubular junction or beyond the sinuses of Valsalva. Consequently, the coronary arteries are upstream of the stenosis and subjected to conditions of systolic hypertension. This situation causes these vessels to dilate, speeds up their atheromatous degeneration, and requires surgery to be performed early in the course of the disease [12,14]. Surgery consists of reconstructing the aortic root with reimplantation of the coronary arteries. This is indicated if the mean pressure gradient is ≥ 50 mmHg or the patient is symptomatic [10].
Bicuspid aortic valve
A bicuspid aortic valve is the most common adult congenital malformation, observed in 1-2% of patients [3]. It is often associated with coarctation of the aorta and more rarely with a perimembranous VSD. Aortic root anomalies linked to it may result in aortic dilation, an aneurysm, or a dissection [1]. A bicuspid aortic valve can be identified by the presence of two cusps rather than three. It is described as a "true" bicuspid aortic valve (type 0) if these two cusps are roughly equal in size with two commissural points and an ellipsoidal opening (Video and Figure 15.49) [9].
Video: Congenital bicuspid aortic valve; the leaflets are in antero-posterior position.

Figure 15.49: Bicuspid aortic valve A: there are only two commissures and the opening (white arrow) is ellipsoidal. B: bicuspid aortic valve in connection with Marfan syndrome; the valve opening is ellipsoidal, the borders of the two cusps do not reach the walls of the aorta during systole.
A bicuspid aortic valve is described as "false" (type 1 and 2) if there are three commissural points and two cusps have secondarily fused leaving a trace of this fusion in the form of a raphe. The opening is banana-shaped (Video). The current classification is based on the number of raphes (see Chapter 11 Bicuspidie aortique) [9].
Video: Calcified bicuspid aortic valve; the right and left coronary cusps are fused together, but there are still 3 commissures.
The bicuspid aortic valve is delicate and its cusps are subjected to more intense stress than a normal valve. When the valve is open, its extremities cannot reach the wall of the sinuses of Valsalva and vibrate during systole, causing a systolic murmur. When it undergoes fibrosis and calcification, it becomes stenotic, generally around the age of 40-50 years (Figure 15.50).

Figure 15.50: Bicuspid aortic valve A: "False" bicuspid aortic valve – there are three commissures (labelled 1,2 and 3), but the opening (white arrow) is ellipsoidal since the non-coronary (NC) and right coronary (RC) cusps are fused at the site of a calcified raphe (red vertical arrow) and only form one cusp. B: Anatomopathological specimen from the case presented in A. LC: left coronary cusp.
The valve can also become insufficient by dilation of the annulus, prolapse, or perforation of a cusp. This generally occurs at an earlier age than stenosis. It can usually be ascribed to aortic disease with variable degrees of stenosis and insufficiency (Video). The more eccentric the opening, the faster the progression. Auscultation of the aortic stenosis reveals a 5/6 ejection murmur with a thrill, a paradoxical split second heart sound and an opening click audible at the apex (for more details, see Chapter 11, Bicuspid aortic valve).
Video: Long-axis view of a bicuspid aortic valve; the colour flow shows a minor regurgitation and a severe stenosis.
Surgical options
In cases of a stenotic bicuspid aortic valve, surgery is indicated if the mean gradient is ≥ 40 mmHg, area ≤ 0.6 cm2/m2, the patient is symptomatic, or the LV is dilating [10,14]. In the event of aortic insufficiency (AI), surgery is indicated based on AI severity and LV dilation. In children and young adults, implantation of a prosthetic valve is avoided as mechanical prostheses require lifelong anticoagulation and biological prostheses only last 10 to 15 years (see Chapter 11 - Prosthetic valves). If the valve is flexible, non-calcified and free of insufficiency, the obstacle may be removed and ventricular remodelling prevented by means of percutaneous balloon valvotomy. However, there is a risk of creating residual insufficiency. The long-term success rate is equivalent to that of a prosthesis [8].
If a valvotomy is inappropriate given the specific circumstances, aortic valve repair (see Chapter 11 Other valve repairs) or a Ross procedure may be performed. This entails transposing the pulmonary valve to the aortic position (with reimplantation of the coronary arteries), and implanting a homograft in the pulmonary position (Figure 15.51; see Figure 11.146) [13].

Figure 15.51: Ross procedure. This entails resecting the diseased aortic valve, implanting the patient's pulmonary valve in this position, and replacing the pulmonary valve with a valved conduit between the RV and PA.
This technique avoids anticoagulation and enables valve growth. The reason for this switch is that the rate of wear for homografts is high in the aortic position (30% at 10 years). Moreover, an incompetence in the pulmonary artery root is easier to manage since the right heart can accommodate major variations in volume; on the other hand, stenosis can be dilated by catheterisation without any risk of systemic embolism. The same wear-related lesions in the aortic position would require the implantation of a prosthesis. The Ross procedure is contraindicated in the event of pulmonary insufficiency, a bicuspid pulmonary valve, or major ventricular dysfunction. The surgical mortality rate for the Ross procedure is 3% and long-term mortality is < 1%/year. While the deterioration rate for the aortic autograft is 1%/year, it is higher for the homograft in the pulmonary artery root (2-3%/year). The neoaortic valve becomes insufficient in 30% of patients [4]. The reoperation rate above the age of 12 years is 10% for the aortic valve and 20-30% for the pulmonary homograft [13]. In patients aged over 40 years, the valve is replaced by a prosthesis, which ideally should be mechanical. Biological prostheses are used only in patients who cannot be anticoagulated or who are aged over 65 years. TAVI-type transcatheter implantation is not appropriate for a bicuspid aortic valve, but may be used for non-invasive treatment of stenosis or pulmonary homograft incompetence [5,6].
Anaesthesia
The requirements applicable to anaesthesia are the same as those for aortic stenosis (see Chapter 11 Aortic Stenosis, Anaesthesia).
- Maintain preload;
- Maintain normal heart rate;
- Maintain systemic perfusion pressure with arterial vasoconstrictors;
- Manage any LV dysfunction;
- In summary, the patient must be: full - regular - tight.
Management is adjusted in the event of aortic insufficiency (see Chapter 11 Aortic insufficiency, Anaesthesia).
- Maintain preload;
- Tachycardia (heart rate of 80 bpm);
- Reduce systemic perfusion pressure with arterial vasodilators;
- Manage dysfunction of the LV, which is dilated.
- In summary, the patient must be: full – fast – open.
In cases of supra-aortic stenosis, blood flow is diverted so that it surges into the brachiocephalic trunk where the pressure is higher than in the distal aortic arch [1]. It is therefore important to insert the arterial catheter in a left radial or femoral position. In reconstructions of the aortic root, the catheter position varies depending on the surgical strategy and CPB cannulation (see Chapter 18, Ascending aorta, Monitoring).
Marfan syndrome
Marfan syndrome is an autosomal dominant disease of the connective tissue whose prevalence is 0.3%. Dilation of the aortic root and ascending aorta is observed in 60-80% of these patients (Videos and Figure 15.52). Dissection is a common complication (see Chapter 18 Ascending aorta) [1].
Video: Massive dilatation of the aortic root in a case of Marfan syndrome.
Video: Aortic regurgitation in the same case of Marfan syndrome.

Figure 15.52: Marfan syndrome A: significant dilation of the aortic root – the aortic valve is bicuspid – it does not open completely during systole. B: severe aortic insufficiency (AI) in a case of Marfan syndrome with dilation of the aortic root.
Left ventricular ejection force must be reduced by a beta-blocker and arterial pressure must be strictly controlled by vasodilators (APsyst < 120 mmHg). Surgery is indicated if the diameter of the ascending aorta is ≥ 5.0 cm (≥ 2.75 cm/m2) or if it increases by > 5 mm/year [1,10,12]. The procedure entails replacing the aortic root (Figure 18.21) and sparing the aortic valve if possible (Figures 18.22 and 18.23) (see Aortic root surgery). Anaesthesia is described in Chapter 18 (Ascending aorta, Anaesthesia).

Figure 18.21: Bentall procedure. The combined prosthesis of a tube graft and a mechanical or bioprosthetic valve is sutured to the aortic annulus, after resection of the aortic valve and the aortic root. The coronary arteries are reimplanted into the graft.
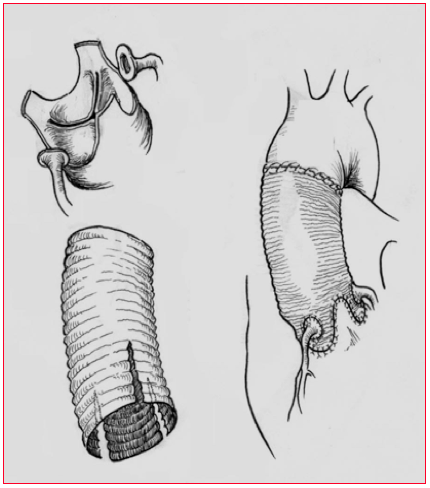
Figure 18.22: Yacoub procedure. The tube graft is carved in order to fit into the sinuses of Valsalva and to maintain the valvular cusps in place. The aortic regurgitation is cured. The coronary arteries are reimplanted into the proximal parts of the graft.

Figure 18.23: Tirone-David procedure. The tubular prosthesis is implanted from outside on the aortic annulus and the commissures of the valve are reimplanted on the inside of the graft. The risk of secondary dilation is less than with the previous operation. The coronary arteries are reimplanted into the graft.
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
Marfan syndrome
Marfan syndrome is an autosomal dominant disease of the connective tissue whose prevalence is 0.3%. Dilation of the aortic root and ascending aorta is observed in 60-80% of these patients (Videos and Figure 15.52). Dissection is a common complication (see Chapter 18 Ascending aorta) [1].
Video: Massive dilatation of the aortic root in a case of Marfan syndrome.
Video: Aortic regurgitation in the same case of Marfan syndrome.

Figure 15.52: Marfan syndrome A: significant dilation of the aortic root – the aortic valve is bicuspid – it does not open completely during systole. B: severe aortic insufficiency (AI) in a case of Marfan syndrome with dilation of the aortic root.
Left ventricular ejection force must be reduced by a beta-blocker and arterial pressure must be strictly controlled by vasodilators (APsyst < 120 mmHg). Surgery is indicated if the diameter of the ascending aorta is ≥ 5.0 cm (≥ 2.75 cm/m2) or if it increases by > 5 mm/year [1,10,12]. The procedure entails replacing the aortic root (Figure 18.21) and sparing the aortic valve if possible (Figures 18.22 and 18.23) (see Aortic root surgery). Anaesthesia is described in Chapter 18 (Ascending aorta, Anaesthesia).

Figure 18.21: Bentall procedure. The combined prosthesis of a tube graft and a mechanical or bioprosthetic valve is sutured to the aortic annulus, after resection of the aortic valve and the aortic root. The coronary arteries are reimplanted into the graft.

Figure 18.22: Yacoub procedure. The tube graft is carved in order to fit into the sinuses of Valsalva and to maintain the valvular cusps in place. The aortic regurgitation is cured. The coronary arteries are reimplanted into the proximal parts of the graft.

Figure 18.23: Tirone-David procedure. The tubular prosthesis is implanted from outside on the aortic annulus and the commissures of the valve are reimplanted on the inside of the graft. The risk of secondary dilation is less than with the previous operation. The coronary arteries are reimplanted into the graft.
| Lesions of the left ejection pathway |
|
Three congenital disorders:
- Membranous sub-aortic stenosis (high incidence of valvular dysplasia and AI) - Bicuspid valve (stenosis and/or insufficiency); - Supra-aortic stenosis. Anaesthetic management is the same as for aortic stenosis: (Full – Regular - Tight) or aortic insufficiency (Full – Fast – Open) depending on the predominant lesion. Marfan syndrome is associated with aortic insufficiency and aneurysmal dilation of the aortic root and ascending aorta with a risk of dissection and rupture. |
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
- BAUMGARTNER H, BONHOEFFER P, DE GROOT NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915-57
- BHATT AB, FOSTER E, KUEHL K, et al. Congenital hesart disease in older adult. A Scientific Statement from the American Heart Association. Circulation 2015; 131:1884-931
- FRIEDMAN WF. Aortic stenosis. In: EMMANOULIDES GC, ed. Moss and Adam's heart disease in infants, children and adolescents including fetus and young adult. Baltimore: Williams & Wilkins 1995, 1087
- FRIGIOLA A, RANUCCI M, CARLUCCI C, et al. The Ross procedure in adults: long-term follow-up and echocardiographic changes leading to pulmonary autograft reoperation. Ann Thorac Surg 2008; 86:482-9
- NISHIMURA RA, OTTO C, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:e521-e643
- NISHIMURA RA, OTTO C, RIGOLIN VH, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017; 70: 252-89
- ROSENFELD HM, GENTLES TL, WERNKOVSKY G, et al. Utility of intraoperative echocardiography in the assessment of residual cardiac defects. Ped Cardiol 1998; 19:346-51
- ROUINE-RAPP K, RUSSELL IA, FOSTER E. Congenital heart disease in the adult. Int Anesthesiol Clin 2012; 50:16-39
- SIEVERS HH, SCHMIDTKE C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133:1228-33
- SILVERSIDES CK, KIESS M, BEAUCHESNE L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol 2010; 26:e80-e97
- STEVENSON JG, SORENSEN GK, GARTMAN DM, et al. Left ventricular outflow tract obstruction: An indication for intraoperative transesophageal echocardiography. J Am Soc Echocardiogr 1993; 6:525-35
- STOUT KK, DANIELS CJ, VALENTE AM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease. J Am Coll Cardiol 2019; 73:e81-192
- TAKKENBERG JJM, KLIEVERIK LMA, SCHOOF PH, et al. The Ross procedure: a systematic review and meta-analysis. Circulation 2009; 119:222-8
- WARNES CA, WILLIAMS RG, BASHORE TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008; 118:2395-451
