Video: Severe mitral insufficiency on dilatation and failure of the LV (MI type IIIb); the mitral leaflets remain almost immobile during the cardiac cycle; the mitral coaptation point is well below the plane of the mitral annulus. Compared with the RV, the LV is very rounded and dilated. Presence of a catheter in the RA.
- Traction on the chords prevents systolic mitral occlusion due to LV dilatation and sphericity (outward displacement of the chords);
- Desynchronisation of papillary contraction;
- Annular dilatation;
- Insufficient contraction force to occlude the mitral valve (occlusion force too low compared to traction force);
- Fibrosis and loss of LV compliance.
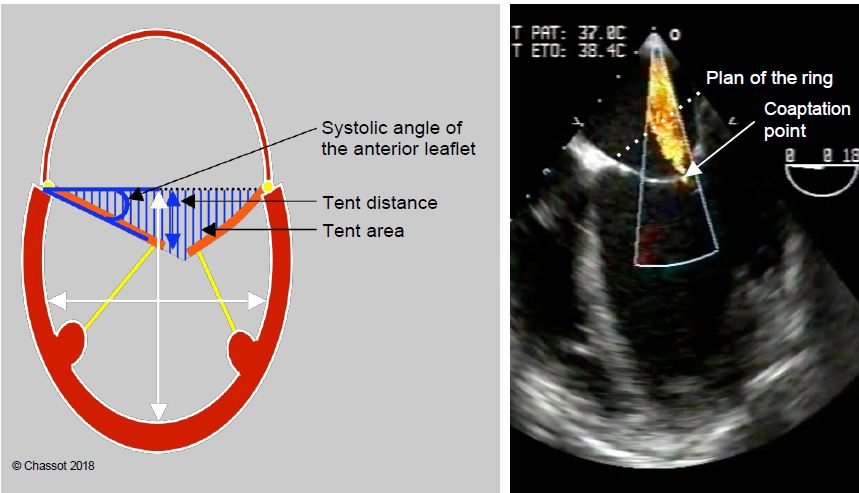
Figure 11.85: Restrictive MI (type IIIb) in a dilated LV. In systole, the leaflets are trapped within the LV by its dilation. The degree of restriction is defined by the distance from the point of coaptation to the plane of the mitral annulus (tenting distance), the triangular area between the leaflets and the plane of the annulus (tenting area), and the angle of closure of the anterior leaflet measured between the plane of the annulus and the tip of the leaflet [15]. The ratio of LV long to short axis diameter defines the degree of sphericity (normal long to short axis ratio: > 1.5).
LV dilatation can affect the mitral valve in two different ways [13,15].
- Proportional MI: There is a linear relationship between regurgitant orifice area and LV dilatation (papillary muscle widening, mitral annulus dilatation and flattening). MI decreases in response to pharmacological treatment of ventricular dilatation or the introduction of ventricular assist devices. However, concomitant plasty does not give favourable results [14].
- Disproportionate MI: The MI is larger than would be predicted by the degree of LV dilatation because of local remodelling or out-of-sync papillary muscle contraction (conduction delay). If the QRS is widened, MI tends to worsen with medical treatment of left-sided dilatation. Resynchronisation and MitraClip™ plasty are effective here [21].
MI occurs in 40-60% of cases of left ventricular failure and is a sign of worsening prognosis [11]. Unless severe, the treatment of MI is primarily that of left ventricular failure (see Chapter 12 Treatment of chronic left ventricular failure).
- Converting enzyme inhibitor (CEI) or angiotensin II receptor antagonist (ARA), sacubitril-valsartan combination;
- Beta blocker;
- Anti-aldosterone (spironolactone, epleronone);
- Resynchronisation with a triple-chamber pacemaker: reducing desynchronisation in papillary muscle contraction reduces MI by up to 50% [7].
Correction of MI in dilatative cardiomyopathy is only indicated if the patient remains symptomatic and the MI progresses despite maximal treatment. It only makes sense if there is some likelihood of reversing ventricular remodelling. If the remodelling or pulmonary hypertension is irreversible, suppressing the MI will not change the prognosis, although it will reduce LV volume overload [3,11]. The less advanced the disease, the better the outcome. The clinical results of recent trials summarise the situation [3].
- In centres with extensive experience, mitral valve repair with a restrictive ring has an operative mortality rate of 1.6-5%; patients' functional capacity and quality of life are improved, but the survival rate remains unchanged: 70% at 2 years, 55% at 5 years [10,18].
- The recurrence rate of 20-30% is high because the progression of the primary disease is not interrupted by the operation; certain echocardiographic features are predictive of recurrence [8].
- Angle of closure of the anterior leaflet with the plane of the mitral annulus > 25°;
- Angle of closure of the posterior leaflet with the plane of the mitral annulus > 45°;
- Distance between the coaptation point and the plane of the mitral annulus > 1.1 mm;
- Tent area between the leaflets and the plane of the mitral annulus > 2.5 cm2;
- EF < 0.25;
- LV Vtd > 250 mL, LV Dts > 4.0 to 4.5 cm (difference between European and American recommendations).
- The operative risk of MVR (6-10%) is higher than that of plasty, but the failure rate is much lower; MVR must respect the subvalvular apparatus because the loss of the LV internal skeleton in the context of ventricular failure leads to massive dilatation.
- When the LV is dilated due to aortic insufficiency (telesystolic diameter 6.9 cm), concomitant correction of a moderate MI by plasty does not affect survival and offers no advantage over simple AVR, as the MI resolves in 88% of cases by correction of the AI alone [9].
- The recently fashionable combination of mitral valve surgery with ventricular restraint or resection and plasty of the dyskinetic zone offers no short-term morbidity or mortality benefit, despite the reduction in ventricular dimensions, reduction in MI and increase in EF, and worsens diastolic function [1,22]. Ventricular reduction performed simultaneously with surgical treatment of coronary ischaemia (CABG) or valvular heart disease (mitral valve replacement) does not reduce mortality or morbidity in patients with left heart failure: the operative mortality rate is 4-6% and the 5-year survival rate is 60% in both groups of patients (CABG alone or CABG + LV reduction + mitral valve replacement) [6].
- The current trend in refractory and decompensated left heart failure is towards ventricular assist devices, which solve the problem of MI.
Percutaneous clipping plasty of the end of each leaflet (MitraClip™ plasty) allows non-invasive reduction of MI (see Endoprosthetic heart valves). When compared to surgical repair, MitraClip™ has a higher technical failure rate (3.2% vs 0.6%, 25-33% residual MI), but significantly lower operative mortality (3.3% vs 16.2%), stroke rate (1.1% vs 4.5%) and bleeding risk (4.2% vs 59%) in high-risk categories [4,16,20]. Current recommendations consider the MitraClip™ appropriate for symptomatic patients with severe primary or secondary MI despite optimal medical therapy, who are considered too high risk for surgery (mortality > 8% for MVR and > 6% for MVP) and who have a life expectancy of at least 1 year [2,12]. Patients with an MI that is disproportionate to the degree of ventricular dilatation are the ones who benefit from clipping [17].
Functional MI in dilatative cardiomyopathy is highly variable depending on haemodynamic conditions. The IPPV acts as a prosthesis for the LV and systemic vasodilatation improves its ejection. Therefore, in GA, regurgitation tends to decrease. When its assessment has a direct therapeutic implication (e.g. unplanned insertion of a support ring), it is of utmost importance to respect two conditions when measuring MI.
- Quantification using techniques that are less dependent on haemodynamics, such as vena contracta diameter, PISA, regurgitant volume calculation or three-dimensional planimetry [5];
- Restoration of baseline conditions: normal SAR (phenylephrine bolus for MAP ≥ 80 mmHg), satisfactory CO (dobutamine infusion), acceptable preload (PCWP ≥ 12 mmHg), ventilator apnoea, no PEEP/CPAP.
Principle of anaesthesia
Both LV ejection and MI are reduced by systemic vasodilation and positive inotropic stimulation. Patients are usually on maximal therapy.
- Remodelling inhibitors: ACE inhibitors, beta blockers;
- Reduction of LV Vtd with vasodilators: ACE inhibitors, nitroprusside, nitroglycerin;
- Inotropic stimulation: dobutamine, milrinone, epinephrine, levosimendan;
- Pacemaker resynchronisation;
- In acute situations: intra-aortic balloon pump; IABP is particularly effective in reducing MI.
Pre-operative treatment should be maintained until surgery and continued intraoperatively (catecholamines, IABP). If possible, the resynchronisation pacemaker should be left in place, as MI worsens immediately if it is interrupted (use bipolar coagulation) [7]. The preferred choice of inotropes is inodilators (dobutamine, milrinone, levosimendan) and myocardial receptors β1, β2 and α stimulants (adrenaline) because of the changes in the receptor population typical of ventricular failure (decrease in in β1, increase in α ).
- Preload: hypervolemia is the rule due to left heart failure and pulmonary congestion; perfusion and saline intake must therefore be restricted as patients suffer from water and salt retention, and nitroglycerine may be added.
- Afterload: must remain low; reduce SAR with nitroprusside, nitroglycerine, isoflurane.
- Contractility: must be supported by positive inotropic agents, often in high doses (dobutamine, milrinone + epinephrine, levosimendan); IABP is very effective in reducing MI.
- Frequency: maintain 70-80 beats/min as flow rate is highly dependent on frequency given fixed stroke volume.
- Pulmonary arterial pressure: lower PAR by hyperventilation in PHT.
- Positive pressure ventilation: beneficial for LV function and reduction of MI, difficult to wean.
- Induction with etomidate.
- Maintain with isoflurane and fentanyl; alternative: infusion of midazolam.
- Restrict fluid and saline intake; give preference to vasoconstrictors and positive inotropes for hypotension.
- IPPVI as required, no early extubation after ECC.
- Special monitoring:
- TEE (changes in MI, LV size and function);
- Pulmonary artery catheter (PAP, PCWP, anterograde stroke volume, SvO2);
- ScO2 (peripheral perfusion).
- For more details, see Chapter 12, Anaesthesia for Ventricular Failure.
| Mitral insufficiency due to LV failure |
|
In dilatative cardiomyopathy, MI is restrictive type IIIb symmetric with LV dilatation (40-60% of left-sided insufficiency). MI can be corrected if:
- Severe symptomatic MI under normal haemodynamic conditions
- Persistence of symptoms and progression of MI despite maximal treatment
- Remodelling and potentially reversible PHT
- Restrictive annuloplasty in a centre where mortality from left-sided failure is ≤ 5%.
The recurrence rate is high (30% at 1 year) and long-term survival is not affected by correction of the MI.
Targeted haemodynamics in functional MI
Reduce preload (stasis hypervolemia)
Low SAR
Increased contractility
Rate 70-80 beats/min
Beneficial LPI
IABP effective in reducing MI
|
References
- ACKER MA, BOLLING S, SHEMIN R, et al. Mitral valve surgery in heart failure: insights from the Acorn clinical trial. J Thorac Cardiovasc Surg 2006; 132:568-77
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- DI SALVO TG, ACKER MA, DEC GW, BYRNE JG. Mitral valve surgery in advanced heart failure. J Am Coll Cardiol 2010; 55: 271-82
- FELDMAN T, CILINGIROGLU M. Percutaneous leaflet repair and annuloplasty for mitral regurgitation. J Am Coll Cardiol 2011; 57:529-37
- GREWAL J, MANKAD S, FREEMAN WK. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009; 22:34-41
- JONES RH, VELAZQUEZ EJ, MICHLER RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med 2009; 360:1705-17
- KANZAKI H, BAZAZ R, SCHZARTZMANN D, et al. A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy. J Am Coll Cardiol 2004; 44:1619-25
- LEE APW, ACKER M, KUBO SH, et al. Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy. Circulation 2009; 119:2606-14
- LIM JY; JONG SH, KIM JB, et al. Management of concomitant mild to moderate functional mitral regurgitation during aortic valve surgery for severe aortic insufficiency. J Thorac Cardiovasc Surg 2014; 148:441-6
- MARWICK TH, STEWART WJ, COSGROVE DM. Mechanisms of failure of mitral valve repair: An echocardiographic study. Am Heart J 1991; 122:149-56.
- MEHRA MR, REYES P, BENITEZ M, et al. Surgery for severe mitral regurgitation and left ventricular failure: what do we really know ? J Cardiac Fail 2008; 14:145-50
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017; 70:252-89
- OBADIA JF, MESSIKA-ZEITOUN D, LEURENT G, et al, MITRA-FR investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379:2297-306
- PACKER M, GRAYBURN PA. Contrasting effects of pharmacological, procedural, and surgical interventions on proportionate and disproportionate functional mitral regurgitation in chronic heart failure. Circulation 2019; 140:779-89
- PHILIP F, ATHAPPAN G, TUZCU EM, et al. MitraClip for severe symptomatic mitral regurgitation in patients at high surgical risk: a comprehensive systematic review. Cathet Cardiovasc Interv 2014; 84:581-90
- PRAZ F, GRASSO C, TARAMASSO M, et al. Mitral regurgitation in heart failure: time for a rethink. Eur Heart J 2019; 40:2189-93
- ROMANO MA, BOLLING SF. Update on mitral repair in dilated cardiomyopathy. J Card Surg 2004; 19:396-400
- RYAN L, JACKSON B, PARISH L, et al. Quantification and localization of mitral valve tenting in ischemic mitral regurgitation using real-time three-dimensional echocardiography. Eur J Cardiothorac Surg 2007; 31:839-4
- 20SORAJJA P, LEON MB, ADAMS DH, et al. Transcatheter therapy for mitral regurgitation. Clinical challenges and potential solutions. Circulation 2017; 136:404-17
- STONE GW, LINDENFELD J, ABRAHAM WT, et al, COAPT investigators. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018; 379:2307-18
- UDELSON JE, STEVENSON LW. The future of heart failure diagnosis, therapy and management. Circulation 2016; 133:2671-86