Main pump is the one providing propulsion of blood at physiological flow (2.4 L/min/m2 ) and normal blood pressure (MAP 60-90 mmHg). Its flow ranges from 100 mL/min to 8 L/min. Two additional pumps are used for suction; one is called left pump (suctioning from the root of the aorta or the LV), the other is called right pump (suctioning in the surgical field). One or two additional pumps are used to infuse cardioplegia solutions. Two models of pumps are used.
- Roller pump: works on the principle of partial occlusion of a flexible tube by rollers; most commonly used. The flow rate depends on the diameter of the tube (usually 1/2 inch in adults), the circumference of the support and the number of revolutions/minute of the pump (50-150 rpm). The stroke volume varies from 12 to 42 mL/rev. The rollers are adjusted to achieve a subocclusion that prevents crushing the blood elements but is sufficient to propel the blood mass against a resistance of 300-400 mmHg. Roller pumps are independent of afterload: they maintain flow regardless of blood pressure. Unfortunately, they can pump air if the venous reservoir is empty, or create cavitation upstream if the inflow is restricted. There are therefore monitoring and control systems to stop them if the pressure is too high (risk of rupture of the tubing) or if air enters the circuit (risk of arterial embolism). Cavitation is caused by a local vacuum (in this case upstream of the occluding roller) which reduces the solubility of dissolved gases in the blood (Figure 7.8).
- Centrifugal pump: propels blood by the action of a rotating turbine (located in a sterile chamber where blood circulates), rotating at high speed (1'000 - 3'000 rpm) and driven by an electromagnet (located in the control console). Two different different models are used: BioMedicus™ (Medtronic, USA), CentriMag™ (Levitronix, Switzerland), RotaFlow™ (Jostra, Germany), Capiox™ (Terumo, USA). The pressure difference between the centre and the periphery created by the centrifugal force inside the cone accelerates the blood that is ejected outside [4]. This type of pump generates little trauma to blood elements and reduces platelet stimulation, but is sensitive to preload and afterload because it is not occlusive: an increase in arterial resistance reduces flow. For an output pressure of 200 mmHg, for example, the pump delivers 4 L/min at 2000 rpm; if the pressure increases to 400 mmHg, the speed must increase to 3,000 rpm to maintain the same flow. When pump is stopped, the arterial circuit must be clamped or blood will flow back. On the other hand, if more than 20-30 mL of air enters the system, the vortex is defused and the centrifugal force drops; the pump tends to stop [2]. It is the safest system for medium-term circulation support (a few days) or for drawing blood from the venous circuit, but its use rate for standard CABG is 30-50% [6,8]. High cost of this product limits use (Figure 7.9).
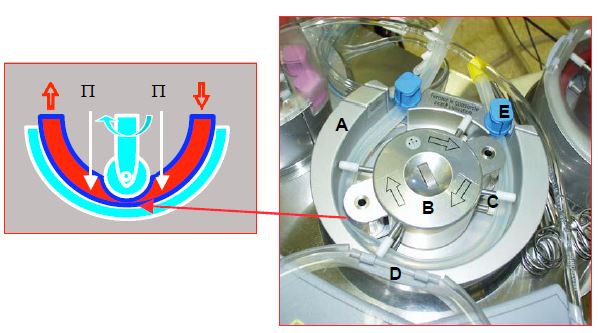
Figure 7.8: Roller pump. A: Pump base plate; the fact that it is about two thirds of the circumference ensures a constant occlusion by the two rollers. B: central hub driving the two rollers, the occlusion of which is adjusted by the knob in the centre. C: 4 pairs of guides keep the circuit in line. D: cover. E: hose clamp (at the pump inlet and outlet). The roller crushes the tube, the rotation propels the blood. This pump is occlusive, which makes it insensitive to afterload, but creates more haemolysis and cavitation due to pressure changes between upstream and downstream of the roller as the rotor compresses the tube. P: pressure in the tube.
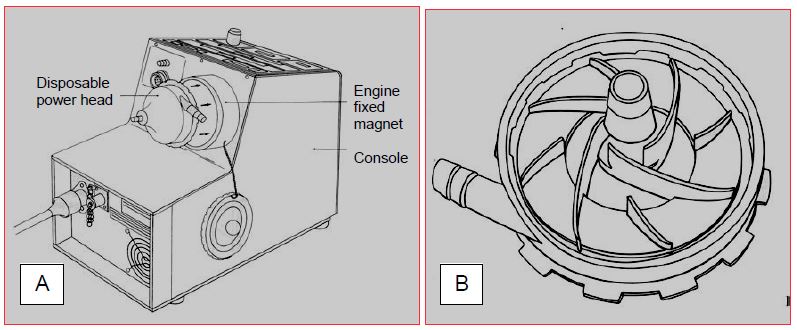
Figure 7.9: Centrifugal pump (BioMedicus™). A: General view of the control console containing the magnetic rotary head driven by an electric motor. B: Bark of the disposable motor head driven by the magnetic rotating head. Blood is propelled by the rotation of the blades. The blood enters through the hub (preload); it is propelled through a tangential orifice, the flow rate of which is sensitive to the afterload. The flow rate of the pump increases by increasing the speed of rotation, but also by increasing the preload and decreasing the afterload.
In the event of motor failure, a manual drive system is used to maintain the blood flow of the ECC. For roller pumps, a hand crank is attached to the rotor. In the case of centrifugal pumps, a manual pump is available as an accessory. In both cases, maintaining the flow requires considerable physical effort. The propulsion of blood by a pump damages the red blood cells and causes some haemolysis. This is proportional to the shear forces experienced by the cells, the pressure gradient between the pump inlet and outlet and the duration of the bypass. It is more important with roller pumps than with centrifugal pumps. This is because roller pumps are relatively occlusive and create a strong pressure gradient between the upstream and downstream of the rollers. The majority of randomised controlled trials give an advantage to centrifugal pumps [6].
Pulsatility
These systems are not pulsatile. Arterial pulsatility transmits more kinetic energy to blood, which improves microcirculation, lymphatic flow and tissue perfusion. The risk of thrombosis is reduced; the neurohumoral stress response is dampened; depulsation-induced vasoconstriction is suppressed; cerebral, coronary and renal perfusion should be improved [1,3]. However, these advantages are outweighed by various shortcomings: technical complexity, pump cost , very high velocity jet during systole, aggravation of haemolysis by a flow rate that is > 10 L/min in systole, damping of pulsatility by the membrane oxygenator and by the arterial cannula, and a flow pattern that bears little resemblance to physiological flow. While experimental results show a clear improvement in capillary perfusion in pulsed mode, clinical data in humans regarding renal, myocardial and neurological function are not significantly different from what is obtained in depulsed mode. Only one randomised study has shown a benefit in mortality and infarct rate [5], but none has reported a difference regarding stroke and renal failure [1]. Current data are too conflicting to make recommendations pro or con the use of pulsatile systems [1]. As the overall benefit of pulsatility is not significant compared to the investment involved, this system is rarely used. Moreover, pulsatility is not necessary for organ perfusion, as demonstrated by the comparison of pulsatile (HeartMate XVE™) and continuous flow (HeartMate II™) ventricular assist systems: 2-year survival is doubled with the depulsed system (24% and 58% respectively) [7]. These results demonstrate that arterial pulsatility becomes optional when the patient is anticoagulated.
| Pumps |
| ECC pumps are of two types:
- Occlusive roller pump, insensitive to load conditions, but causing haemolysis; risk of cavitation and air propulsion - Centrifugal pump, less traumatic and less emboligenic, but sensitive to conditions of charge Pulsatility appears to be optional in an anticoagulated patient; it presents more mechanical and haemolytic risks than continuous flow. There is insufficient data to make recommendations pro or con pulsatile pumps. |
© CHASSOT PG, GRONCHI F, last update December 2019
References
- ALGHAMDI AA, LATTER DA. Pulsatile versus nonpulsatile cardiopulmonary bypass flow: an evidence-based approach. J Card Surg 2006; 21:347-54
- HESSEL EA. Cardiopulmonary bypass equipment. In: ESTEFANOUS FG, et al. Cardiac anesthesia. Principles and clinical practice, 2nd edition. Philadelphia, Lipincott Williams & Wilkins, 2001, 335-86
- HORNICK P, TAYLOR K. Pulsatile and non-pulsatile perfusion: the continuing controversy. J Cardiothorac Vasc Anesth 1997; 11: 310-6
- ISETTA C, CADUSSEAU JL. Extracorporeal circulation circuits: reusable materials, single-use materials. In: JANVIER G, LEHOT JJ (ed). Circulation extracorporelle: principes et pratique, 2ème édition. Paris, Arnette Groupe Liaison SA, 2004, 59-86
- MURKIN JM, MARTZKE JS, BUCHAN AM, et al. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery ECC. I Mortality and cardiovascular morbidity. J Cardiothorac Vasc Surg 1995; 110:340-48
- MURPHY GS, HESSEL EA, GROOM RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009; 108:1394-417
- SLAUGHTER MS, ROGERS JG, MILANO CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241-51
- WARREN OJ, WATRET AL, DE WIT KL, et al. The inflammatory response to cardiopulmonary bypass: Part 2 - Anti-inflammatory therapeutic strategies. J Cardiothorac Vasc Anesth 2009; 23: 384-93