- Obstructive cardiomyopathy (HOCM) or asymmetric hypertrophic cardiomyopathy with predominant hypertrophy of the interventricular septum is a primary myocardial disease leading to dynamic muscular obstruction of the outflow tract (HISS; hypertrophic idiopathic subaortic stenosis; HOCM, hypertrophic obstructive cardiomyopathy) (see Chapter 13 Obstructive Cardiomyopathy).
- The HOCM effect, or dynamic subaortic stenosis, is secondary to concentric left ventricular hypertrophy and certain haemodynamic conditions.
HOCM is a hypertrophic cardiomyopathy characterised by asymmetric thickening of the septum in the LV outflow tract (LVOT). However, in two-thirds of cases it is associated with mitral leaflet elongation (34 mm instead of 23-25 mm for the anterior leaflet) and anterior displacement of the papillary muscles [6,9]. Combined with the muscular stenosis of the LVOT, the anterior tilt of the distal part of the anterior leaflet (SAM, see below) leads to mesosystolic subobstruction of the left outflow tract, producing a maximum gradient of approximately 50 mmHg.
Four conditions must be met for a "HOCM effect" to occur (see Figure 13.9 on page 112 and Figure 11.122).
- Left ventricular hypertrophy of the concentric type, with a strong muscle and a narrow outflow tract; EF is ≥ 50%.
- Anterior mitral leaflet is long and flexible.
- Exaggerated decrease in LV telesystolic volume due to severe hypovolemia or drastic decrease in afterload (vasoplegia, removal of aortic stenosis after AVR); LV tends to collapse in telesystole.
- Excessive sympathetic stimulation that increases the concentric stroke of the posterior wall in systole and moves the coaptation point of the mitral valve anteriorly. It is caused by too rapid administration of beta-catecholamines and is exacerbated by light anaesthesia .
A number of preoperative factors are risk markers for SAM (see Figure 11.49) [10].
- EF > 60% and reduced left ventricular cavity size;
- Posterior leaflet height > 15 mm;
- Reduced aorto-mitral angle (< 140°);
- Distance between interventricular septum and mitral coaptation point (C-sept) reduced (< 2.5 cm);
- Anterior displacement of the anterolateral papillary muscle.
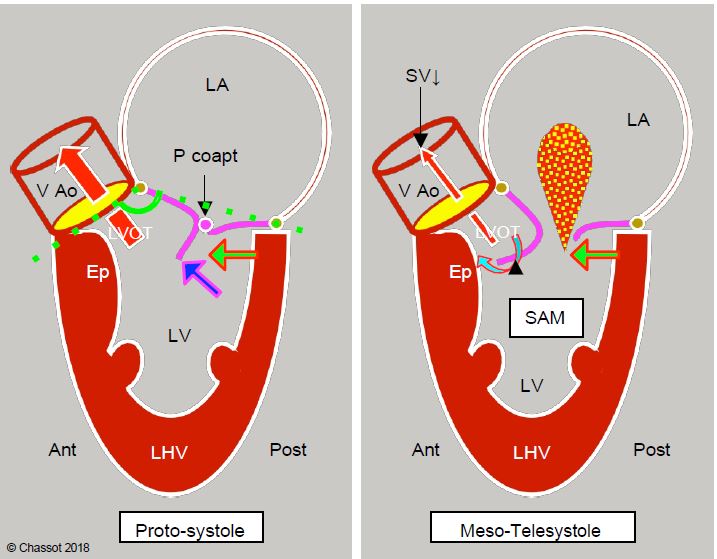
Figure 11.122: Dynamic subaortic stenosis in hypertrophic cardiomyopathy. Concentric hypertrophy and ventricular cavity narrowing (often exacerbated by hypovolaemia, reduced afterload or beta inotropic overstimulation) displace the posterobasal part of the LV anteriorly (green arrow). In the protozoan, the anterior leaflet and the coaptation point (Pcoapt) of the mitral valve are displaced towards the outflow tract (LVOT) and the angle between the plane of the aortic valve and that of the mitral valve closes (mitro-aortic angle: green dotted line). The coaptation point is located between the edge of the posterior leaflet and the body of the anterior leaflet, with the distal part of the anterior leaflet floating within the LV (blue arrow). At the start of systole, intraventricular pressure pushes the anterior leaflet into the LVOT instead of against the posterior leaflet. The acceleration of the flow in the LVOT then creates a Venturi effect, which secondarily sucks in the anterior leaflet of the mitral valve (SAM). In mesotelesystole, the mitral leaflet contributes to the dynamic obstruction of the LVOT. As the mitral valve is no longer occluded, mitral regurgitation (MI) occurs in the second half of systole. Ep: hypertrophied septal spur. LVH: Left ventricular hypertrophy. SAM: systolic anterior motion. VAo: aortic valve.
When the left ventricular cavity becomes very small, the posterobasal wall of the LV is displaced anteriorly and the mitral coaptation zone moves closer to the ejection zone. This is because the posterior part of the mitral annulus is thin and flexible, whereas the anterior part is attached to the fibrous trine, which is the mechanical centre of gravity of the heart and to which the mitral, aortic and tricuspid valves are anchored. It is therefore the posterior zone that moves forward when the LV becomes too narrow. The reduction in the size of the ventricular cavity therefore results in an anterior displacement of the posterior structures. This movement has several effects in systole (see Figure 11.122 above) [4].
- Closure of the mitral valve angle, usually > 150°;
- Anterior displacement of the mitral coaptation zone;
- Mitral coaptation between the distal end of the posterior leaflet and the body of the anterior leaflet rather than edge to edge;
- Part of the anterior leaflet distal to the point of coaptation floating in the LV cavity;
- Increased travel of the posterior abutment chords allowing greater anterior displacement of the leaflets.
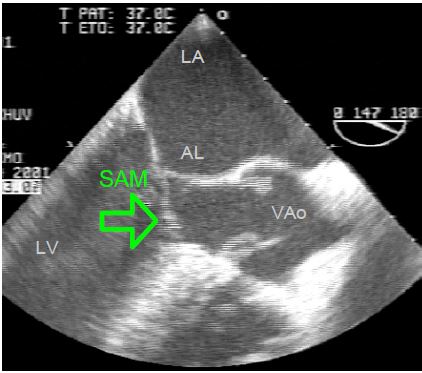
During systole, intraventricular pressure pushes the distal part of the anterior leaflet forward towards the LVOT, where ejection has begun. As Vmax increases in the LVOT, the distal part of this leaflet can be sucked in by the Venturi effect, causing a phenomenon known as systolic anterior motion (SAM): the anterior leaflet bends and partially occludes the LVOT (Figure 11.123). This is the HOCM effect, analogous to the mechanism of obstructive cardiomyopathy [5]. It is clearly visible on TEE views of the LVOT (5-cavity 0-30° and long-axis 120-140°).
Video: Anterior systolic shift ( SAM) of the anterior mitral leaflet in the mesosystolic flow chamber (140° long-axis view); this HOCM effect is linked to an episode of hypovolaemia in a patient with concentric left ventricular hypertrophy.
Video: Anterior systolic shift (SAM) of the anterior mitral leaflet in the mesosystolic pressure chamber (130° long-axis view); this HOCM effect occurs after aortic replacement for stenosis with a bioprosthesis.
Video: Anterior systolic shift ( SAM) of the anterior mitral leaflet in the mesosystolic pressure chamber (4-cavity 0° view); this HOCM effect occurs after aortic replacement for stenosis.
Figure 11.123: Dynamic subaortic stenosis. TEE image of systolic anterior motion (SAM) in the LVOT (140° long-axis view).
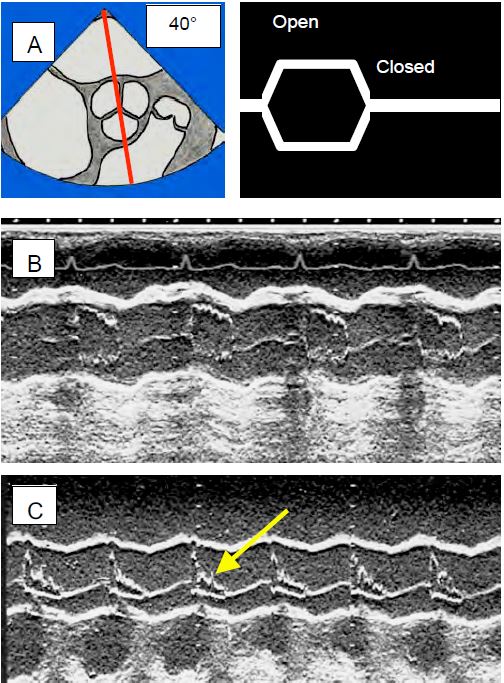
SAM produces flow acceleration > 2.5 m/s and a significant pressure gradient within the LVOT; the systolic flow rate drops abruptly [7]. This mesosystemic drop in flow leads to premature collapse of the aortic valve leaflets as the ejected volume is suddenly insufficient to keep them open (Figure 11.124). This sign, clearly visible through the aortic valve in TM mode, is pathognomonic of the HOCM effect. As long as it is present, the phenomenon is active and requires aggressive treatment; only its disappearance indicates therapeutic success. However, when the valve is replaced by a prosthesis, this element is not visible. All that can be seen is the change in the silhouette of the aortic flow, which is deformed into a "dagger" shape. This mesosystolic fall in aortic flow is often visible on the arterial trace, which shows the same notch in the ascending part of the trace (Figure 11.125).
Figure 11.124: Echocardiographic views of the aortic valve in TM (time motion) mode. The decrease in stroke volume causes the aortic valve leaflets to collapse because the decrease in volume no longer keeps them open. A: TM axis through a short-axis view of the aortic valve; diagram of the opening and closing of the aortic valve. B: The normal image is almost square, the valve is open throughout systole. C: The image in HOCM shows collapse of the leaflets (arrow) in mesosystole. This appearance is pathognomonic of subocclusion of the LVOT.
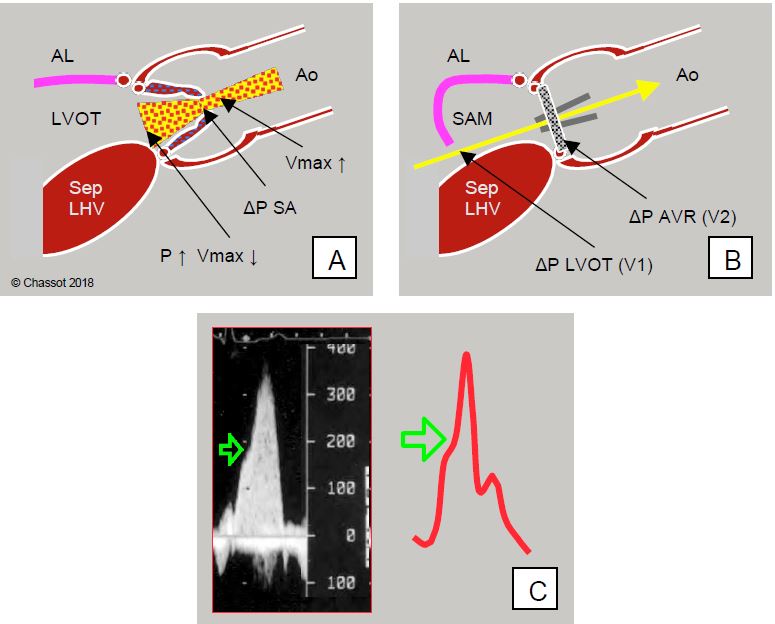
Figure 11.125: Dynamic subaortic stenosis after aortic valve replacement (AVR). A: Before AVR, maximum velocity (Vmax) across the aortic stenosis is high (> 4 m/s), but not in the chase chamber (LVOT) (< 1 m/s) because it is slowed down before the valve obstruction; the LV and interventricular septum are hypertrophic (Sep LVH). B: After AVR, the obstruction is removed and Vmax across the valve decreases, but the LVOT remains hypertrophic and may now narrow excessively during systole in response to sympathetic stimulation, since there is no obstruction to maintain high pressure. Excessive Vmax in the LVOT sucks in the anterior leaflet (AL) of the mitral valve, which partially obstructs the LVOT (SAM: systolic anterior motion). This dynamic stenosis represents a significant pressure gradient (ΔP), which is added to the own ΔP of the prosthesis when the gradient is measured on Doppler echo (yellow arrow). C: Dynamic subocclusion of the LVOT occurs in the second part of systole and is manifested by a decrease in flow but an acceleration of the flow; this phenomenon is expressed by a notch (green arrow) on the upward slope of the flow, often visible on the blood pressure waveform (dagger deformation).
The aspiration of the anterior leaflet into the LVOT has the secondary effect of reopening the mitral valve in mesosystole and causing meso-tesystolic insufficiency. As a result of this MI, the PCWP read on the pulmonary catheter is deceptively high despite ventricular hypovolaemia.
After AVR, the conditions for a HOCM effect are often present: there is concentric LVH, the afterload has fallen because the stenosis has been relieved, hypovolaemia is common on weaninf off pump, and the administration of catecholamines forces contraction of the posterior wall, narrowing of the LVOT and blood ejection velocity. The SAM phenomenon occurs at the end of bypass in 14% of AVR for stenosis [1]. It is confirmed by echocardiography: the ventricular cavity is small, Vmax in the outflow chamber is greater than 2.5 m/sec, the gradient is > 30 mmHg in moderate cases and > 50 mmHg in severe cases, the mitral leaflet falls back into the outflow chamber (SAM), mitral insufficiency occurs in the second half of systole [2,3]. It is essential to calculate the transvalvular gradient using Bernoulli's equation, which takes into account the velocity in the plenum: ΔP = 4 · (Vtotal2 - VLVOT2 ), because the latter (VLVOT ) is much higher than 1.5 m/s; it alone gives a ΔPmax > 25 mmHg, which must be subtracted from the total gradient of the ejection tract (Vtotal) to measure the gradient specific to the aortic prosthesis (see Figure 11.121). The ratio between the velocity in the LVOT and that across the valve (VTILVOT / VTIVao) is useful in assessing the degree of acceleration generated by the prosthesis; normally this ratio should be > 0.5 [12].
- Decreased contractility: discontinuation of catecholamines β ;
- Increase in ventricular volume: infusion of crystalloids or colloids;
- Increase afterload: arterial vasoconstrictors with a pure alpha effect (phenylephrine, noradrenaline);
- Deepening anaesthesia;
- If these measures are insufficient: esmolol (repeated boluses of 10 mg), prescription of β-blocker in the post-operative period;
- In short, in the case of HOCM, the patient must be kept full, slack and closed.
- Post-AVR: surgical resection of the LVOT musculature (myectomy), especially at the level of the septal spur. Care must be taken with this myectomy as excessive depth of excision may result in an interventricular septal defect that requires immediate repair.
- Post-mitral plasty: Repair of the posterior leaflet plasty to reduce its length, possibly changing the ring to a larger size; the aim is to move the coaptation point of the valve further posteriorly and away from the LVOT [11].
- Obstructive cardiomyopathy; alcohol embolisation of the first septal artery to induce partial necrosis of the muscle, surgical septal myectomy, shortening plication of the anterior leaflet, repositioning of the anterior papillary muscle [9].
- Diagnosis of SAM:
- Tilting of the distal part of the anterior mitral leaflet in the LVOT during mesosystole (mid-esophageal 4-cavity 0° and long-axis 120° views).
- Flow acceleration in the LVOT (colour flow aliasing, Vmax > 2.5 m/s, ΔP > 25 mmHg); "dagger" silhouette of transaortic spectral flow (transgastric views).
- Mesosystemic collapse of the aortic cusps (TM mode of the aortic valve in short axis 40°).
- Meso-tele-systolic mitral regurgitation (short duration) directed posterolaterally.
- Measurement of mitral leaflet length.
- Measurement of septal thickness at the LVOT and septal spur.
- Identification of markers for SAM risk.
- Search for post-operative VSD.
| Dynamic subaortic stenosis |
|
The combination of concentric LVH, a long and flexible anterior mitral leaflet, hypovolaemia, reduced afterload and inotropic overstimulation can lead to dynamic obstruction of the LVOT, identical to the mechanism of obstructive cardiomyopathy (HOCM). The result is a mesosystolic subobstruction of the LVOT by the distal part of the anterior leaflet (SAM, systolic anterior motion). The phenomenon is characterised by
- Vmax > 2.5 m/s in the LVOT
- Suboclusion of the LVOT by the anterior leaflet of the mitral valve (SAM)
- Mesosystemic collapse of the aortic valve leaflets (short or long axis of the aortic valve).
- Meso-Telesystolic MI
- Dagger-shaped aortic flow and arterial curve
The result is an episode of hypotension and low flow, aggravated by catecholamines.
Treatment:
- Reduce catecholamines β , bolus esmolol
- Increased preload (infusions)
- Increase afterload ( α vasoconstrictors)
- If unsuccessful: return to ECC for LVOT myectomy
Investigate haemodynamics in case of HOCM effect
High preload
Decreased contractility (stop catecholamines β , esmolol)
Systemic vasoconstriction
Full - slack - closed
|
References
- BARTUNEK J, SYS SU, RODRIGUEZ AC, et al. Abnormal systolic intraventricular flow velocities after valve replacement for aortic stenosis. Circulation 1996; 93:712-9
- GERSH BJ, MARON BJ, BONOW RO, et al. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary. Circulation 2011; 124:2761-96
- HENSLEY N, DIETRICH J, NYHAN D, et al. Hypertrophic cardiomyopathy: a review. Anesth Analg 2015; 120:554-69
- JAIN P, PATEL PA, FABBRO M. Hypertrophic cardiomyopathy and left ventricular outflow tract obstruction: expecting the unexpected. J Cardiothorac Vasc Anesth 2018; 32:467-77
- LEFEBVRE XP, HE S, LEVINE RA, et al. Systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy: an in vitro pulsatile flow study. J Heart Valve Dis 1995; 4:422-38
- LEVINE RA, HAGÉGE AA, JUDGE DP, et al. Mitral valve diasease – morphology and mechanisms. Nat Rev Cardiol 2015; 12:689-710
- LIN CS, CHEN KS, LIN MC, et al. The relationship between systolic anterior motion of the mitral valve and the left ventricular outflow tract Doppler in hypertrophic cardiomyopathy. Am Heart J 1991; 122:1671-6
- MASLOW AD, HAERING JM, LEVINE RA, et al. Echocardiographic predictors of left ventricular outflow tract obstruction and systolic anterior motion of the mitral valve after mitral valve reconstruction for myxomatous valve disease. J Am Coll Cardiol 1999; 34:2096-104
- SHERRID MV, BALARAM S, KIM B, et al. The mitral valve in obstructuve hypertrophic cardiomyopathy. A test in context. J Am Coll Cardiol 2016; 67:1846-58
- TAVLASOGLU M, DURUKAN AB, GURBUZ HA. Is a "narrow aorto-mitral angle and associated factors" associated with development of systolic anterior motion? J Thorac Cardiovasc Surg 2013; 145:617
- VARGHESE R, ANYANWU AC, ITAGAKI S, et al. Management of systolic anterior motion after mitral valve repair: an algorithm. J Thorac Cardiovasc Surg 2012; 143:S2-7
- ZOGHBI WA, CHAMBERS JB, DUMESNIL JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocradiogr 2009; 22:975-1014