The various aspects of ventricular support are detailed in Chapter 12 (see Ventricular Support). Here we will only discuss intraoperative support situations that use, extend or replace a bypass machine. Extracorporeal support is indicated when the heart can no longer provide haemodynamics that meet the needs of the body and the situation must be stabilised by a mechanical device to avoid the drama of multi-organ failure. An oxygenator can be added when the lungs no longer provide gas exchange. The prognosis should be one of four possibilities: 1) momentary support during surgery, 2) likely myocardial recovery within days or weeks, 3) waiting for a transplant, or 4) anticipating long-term support [15]. Several techniques are available (Table 7.18).
- Without oxygenator:
- Intra-aortic counterpulsation (IACP) (see Chapter 12, IACP);
- Ventricular assist via percutaneous (Impella™, TandemHeart™) or central (CentriMag™, Abiomed™) cannulation (see Chapter 12, Short-Term Devices);
- With oxygenator:
- ECMO (extracorporeal membrane oxygenation) or ECLS (extracorporeal life support) (see Chapter 12, ECMO).
There are many indications for assistance [1,14].
- Failed weaning off from ECC (survival rate 25-35%) [7];
- Stabilisation before cardiac surgery (ventricular failure, massive mitral insufficiency, active ischaemia, IVC);
- Cardiogenic shock (massive infarction, cardiac contusion);
- Fulminant myocarditis;
- Complex PCI (common trunk) in case of severe left dysfunction;
- Sepsis and circulatory collapse;
- Drug intoxication (anti-arrhythmics such as flecainide or digitalis); haemofiltration accelerates clearance;
- Pulmonary embolism;
- Post-hospital cardiac arrest (2-4 fold improvement in survival) [12];
- Bridging to long-term assistance.
Placement is performed under fluoroscopic and echocardiographic control. These systems require complete anticoagulation with heparin (ACT > 300 seconds). Their performance depends on several conditions [8].
- Functional RV; continuous inotropic support is often required to sustain the RV;
- Good gas exchange;
- Absence of aortic insufficiency (AI grade > I) (risk of acute LV dilation);
- Good size femoral vessels (risk of ischaemia in the cannulated limb).
The contraindications are important: age > 75 years, life expectancy < 1 year, severe liver disease, impossible anticoagulation, irreversible neurological damage, incurable neoplasia [15].
Approximately 10% of patients experience problems with the cannulation. mainly because of limb ischaemia in which the arterial cannula is implanted. To avoid this complication, a small bypass cannula vascularises the limb which artery is occluded by the main cannula [3].
ECMO
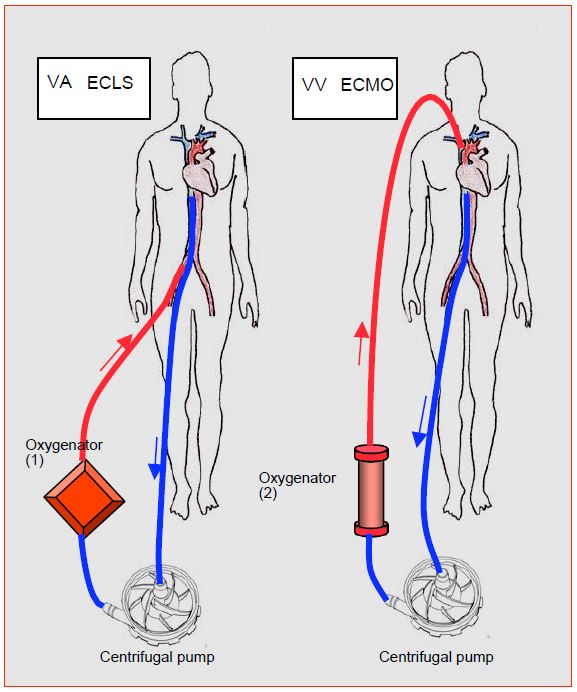
ECMO is certainly the first option in cardiorespiratory failure [15]. Venous blood is drained/sucked to a centrifugal pump and propelled through a membrane oxygenator and returned to the patient via an arterial cannula (femoral, axillary, subclavian). This is the veno-arterial mode, used in cases of primary circulatory failure. If the patient is suffering exclusively from respiratory failure, a veno-venous circuit is adopted which provides gas exchange but no haemodynamic support (Figure 7.56).
Figure 7.56: ECLS (Extra-corporeal life support) and ECMO (Extra-corporeal membrane oxygenation) circuits. 1: Veno-arterial (VA) circuit. Blood is drawn from the inferior vena cava in the vicinity of the RA through a femoral cannula; it is returned under arterial pressure to the femoral artery after oxygenation; the support is cardiorespiratory. In cardiac surgery, the cannulas can be introduced centrally (RA and aorta). The right and left heart contain only venous blood. 2: veno-venous circuit (VV). Blood is collected in the same way, but returned under low pressure to a large central vein, in this case the internal jugular. The support is respiratory [13]. The right and left hearts contain mixed venous blood (saturated and desaturated). The oxygenator is most often a micropore tubular type made of polymethylpentane. The roller pumps, identical to those used in ECC, are abandoned because of their excessive haemolysis when used beyond a few hours.
The oxygenator is of the polymethylpentane micropore type, which unfortunately impedes the passage of halogens but operates for several days without changing its characteristics. ECMO requires anticoagulation with non-fractionated heparin to achieve an anti-Xa activity of 0.3-0.5 IU/mL [10]. ECMO increases LV afterload due to its retrograde arterial flow. It is therefore important to monitor the LV on echocardiography. If the aortic valve never opens, if the ventricle dilates and if the arterial curve shows no pulsatility, ECMO flow should be reduced, LV drainage inserted and/or mechanical ventricular support provided [10]. The weaning rate with long-term survival is on average slightly above 60% [6]. The list of complications is long: ischaemia of the cannulated limb, thromboembolism, stroke, haemorrhage, haemolysis, infection, aortic insufficiency [15].
Ventricular assistance
It is monoventricular and is used to maintain organ perfusion during an episode of cardiogenic shock, or to perform high-risk percutaneous interventions under the cover of a circulatory prosthesis [4]. These systems are designed to operate for a short period of time, up to 2-4 weeks depending on the model [8]. They are implantable via femoral cannulation (percutaneous systems) or central access (LA, LV, ascending aorta). Pulmonary flow and gas exchange are usually provided by the patient's own RV and lungs. These devices are indicated in circumstances where the left heart can no longer sustain life. They provide only mean aortic pressure since are not pulsatile and propel a continuous flow; in this case, the lack of PAs may compromise coronary systolic perfusion of the RV, especially in case of right-sided dysfunction and/or pulmonary hypertension. Some can be implanted as right-sided support in the event of RV failure. Figure 7.57 shows two examples of such devices.
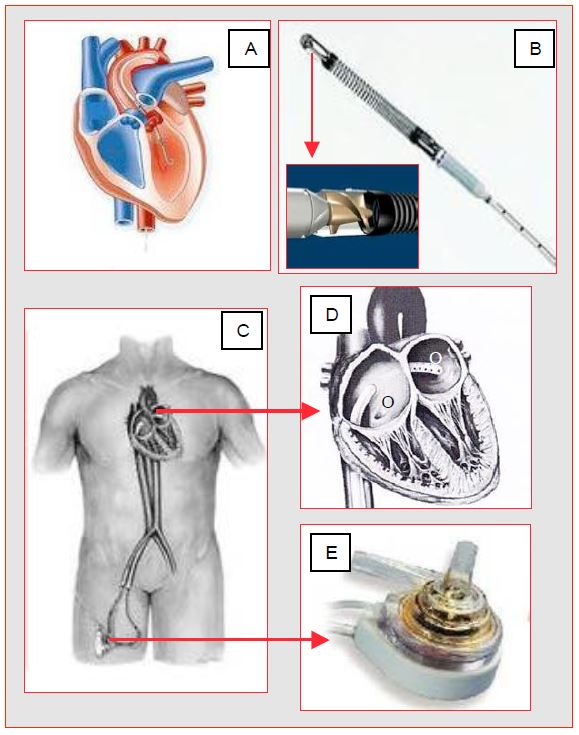
Figure 7.57: Percutaneous left ventricular assist systems. A: Impella™; the cannula introduced through the femoral artery or ascending aorta (if sternotomy) straddles the aortic valve. The internally rotating Archimedean screw (B) draws blood from the LV and returns it to the ascending aorta. C: TandemHeart™; the femorally inserted venous cannula draws blood from the LA across the interatrial septum (D); the blood is returned by a centrifugal pump (E) into the femoral artery.
- The Impella™ consists of an Archimedean screw mounted in a catheter placed through the aortic valve; it draws blood from the LV and returns it to the ascending aorta. The system can also be used for right single ventricular support between RV and pulmonary artery. The placement of the cannula astride the aortic valve is very precise, otherwise the valve may become insufficient or the pump flow may dilate the LV; transoesophageal echocardiography is very useful to check its adequacy. Specific contraindications: aortic insufficiency or stenosis, aortic valve prosthesis, LV thrombus.
- The TandemHeart™ drains venous blood from the RA through a trans-septal cannula (21F) introduced via the femoral route and returns it to the femoral artery (cannula 15-17F) by means of a centrifugal pump that delivers up to 4 L/min at 7'500 rpm.
Compared to ECMO, ventricular assist systems provide better cardiac output, higher systemic pressure and lower PAPO, but with little impact on long-term survival [2,5]. The possibility of managing refractory circulatory arrest with easy-to-install ventricular support raises a major issue: how to assess the chances of long-term recovery of cardiac and neurological function? Two criteria should be considered: 1) the duration of no-flow, and 2) the duration of low-flow. The following can be considered as a possible indication for circulatory support [9]:
- Zero cardiac output time < 5 minutes;
- Duration of low cardiac output (in resuscitation) < 100 minutes and PeCO2 > 10 mmHg.
It is obvious that these simple criteria must be weighed against the circumstances of the cardiac arrest (age, trauma, hypothermia, etc). They address in-hospital arrests.
Internally cannulated systems (CentriMag™, Abiomed™, Thoratek™ PVAD) involve a sternotomy to introduce the drainage cannula into the LV apex or LA and attach the ejection cannula to the ascending aorta; this anastomosis between the tubular prosthesis and the anterior aorta involves tangential clamping of the latter.
Consequences for anaesthesia
Although some percutaneous systems can theoretically be implanted under sedation, general anaesthesia is required for placement of support (see Chapter 12 Anaesthesia and ventricular support). An arterial catheter is mandatory, as rotary or axial pumps provide a depressed pressure, equivalent to MAP; in addition, pulse oximetry is inoperative. The arterial cannulation site is chosen according to the cannulations of the assist; it is better radial in femoral cannulation, in order to measure the pressure at the end of the circuit and the SaO2 of the oxygenated blood by the lungs in case of Harlequin syndrome (see Chapter 12 ECMO). A central venous line is also necessary for monitoring right-sided dysfunction (CVP > 12 mmHg) and for the administration of inotropic agents; a Swan-Ganz catheter is very useful, but it is not always possible to insert in challenging conditions as emergency and deep cardiogenic shock. In situations where clock’s ticking, minimal monitoring is often required. Transoesophageal echocardiography (TEE), on the other hand, is easy to set up and provides essential information on the aortic valve (absence of AI), cannula position, LV unloading and LV function [11]. Oxygenation is monitored by SaO2 and SvO2 measured on the arterial and venous cannulae, respectively.
The onset of left-sided monoventricular support can still lead to acute right-sided failure because RV dysfunction was covered by LV dysfunction and because the pump suddenly increases systemic venous return to the right. On the other hand, decompression of the LV changes the geometry of the interventricular septum and negates its participation in right ejection. This threat of right-sided failure means that steps must be taken to support the RV before cardiogenic shock occurs.
- Hyperventilation with a medium Pit maintained as low as possible;
- Inotropic agent infusion (milrinone + adrenaline, levosimendan);
- Perfusion of noradrenaline or vasopressin to maintain interventricular septal position and coronary perfusion pressure;
- Pulmonary vasodilator: NO, prostacyclins, nitroglycerin.
Should these measures prove insufficient, a right-sided assistance must be installed.
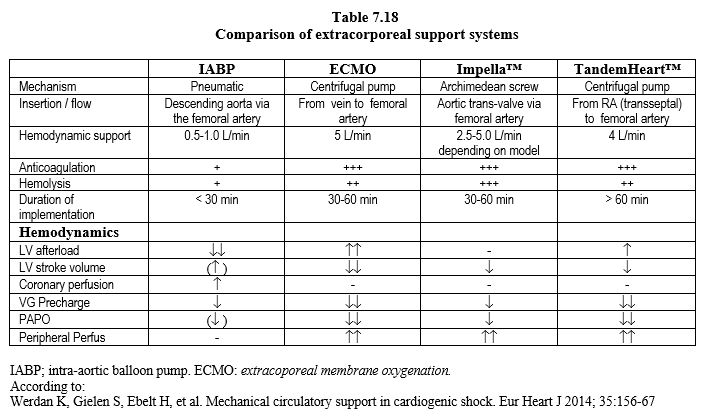
| Ventricular assist systems |
|
Percutaneous left assist systems (femoral route):
- ECMO: femoral vein→ centrifugal pump→ oxygenator→ artery (femoral, subclavian, axillary)
- Impella® : Archimedean screw straddling the aortic valve (2.5 - 5.0 L/min), pumps blood into the LV and propels it into the ascending aorta
- TandemHeart® : pumps blood into the LA through a trans-septal cannula and into the femoral artery
Central cannulation (sternotomy) left assist systems: CentriMag™, Abiomed™, Thoratek™ PVAD.
The initiation of a left asistance can trigger a right failure.
These devices are non-pulsatile and give a linear MAP with overlayed LV systolic ejection.
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- BIRNBAUM DE. Extracorporeal circulation in non-cardiac surgery. Eur J Cardiothorac Surg 2004; 26:S82-5
- CHENG JM, DEN UIL CA, HOEKS SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: A meta-analysis. Eur Heart J 2009; 30:2102-8
- MOHITE PN, FATULLAYEV J, MAUNZ O, et al. Distal limb perfusion: Achilles' heel in peripheral venoarterial extracorporeal membrane oxygenation. Artif Organs 2014; 38:940-4
- NAIDU SS. Novel percutaneous cardiac assist devices. The science of and indications for hemodynamic support. Circulation 2011; 123:533-43
- OUWENEEL DM, ERIKSEN E, SJAUW KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2017; 69:278-87
- PADEN ML, CONRAD SA, RYCUS PT, et al. Registry. Extracorporeal Life Support Organization Registry report 2012. ASAIO J 2013; 59:202-10
- POKERSNIK JA, BUDA T, BASHOUR CA, et al. Have changes in ECMO technology impacted outcomes in adult patients developing postcardiotomy cardiogenic shock? J Card Surg 2012; 27:246-52
- PULIDO JN, PARK SJ, RIHAL CS. Percutaneous left ventricular assist devices: Clinical uses, future applications, and anesthetic considerations. J Cardiothorac Vasc Anesth 2010; 24:478-86
- RIOU B, ADNET F, BAUD F, et al. Recommendations on the indications of circulatory assistance in the treatment of refractory cardiac arrest. Ann Fr Anesth Réanim 2009; 28:182-6
- SCHAHEEN BW, THIELE RH, ISBELL JM. Extracorporeal life support for adult cardiopulmonary failure. Best Pract Res Clin Anaesthesiol 2015; 29:229-39
- SCHWARZ B, MAIR P, MARGREITER J, et al. Experience sith percutaneous venoarterial cardiopulmonary bypass for emergency circulatory support. Crit Care Med 2003; 31:758-64
- SHIN TG, JO IJ, SIM MS, et al. Two-year survival and neurological outcome of in-hospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. Int J Cardiol 2013; 168:3424-30
- SIDEBOTHAM D, ALLEN SJ, McGEORGE A, et al. Venovenous extracorporeal membrane oxygenation in adults: Practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth 2012; 26:893-909
- SUBRAMANIAN B. Mechanical circulatory support. Best Pract Res Clin Anaesthesiol 2015; 29:203-27
- VAN DIEPEN S, KATZ JN, ALBERT NM, et al. Contemporary management of cardiogenic shock. A scientific statement from the American Heart Association. Circulation 2017; 136:e232-e268