Platelets are anucleate corpuscles with a life span of 7-10 days. Produced in the marrow, they are eliminated by the reticuloendothelial system in spleen and liver. Platelets are closely dependent on red blood cells for their supply of thromboxane or prostaglandin precursor lipids and substrate for ADP metabolism [11]. In addition, marginalisation of platelets in blood stream by mass of erythrocytes that remain in the middle of the stream increases the chances of thrombocyte attachment to the vascular wall [17]. In severe anaemia, erythocyte transfusion therefore improves platelet function.
Platelet transfusion
One unit of platelets contains about 2 x 1011 thrombocytes. In an adult, it increases the circulating level to a maximum of 20,000/mcL, often less. Most surgical procedures can be performed without difficulty with a thrombocyte count between 50,000/mcL and 75,000/mcL; only intracranial procedures require a value of > 100,000/mcL. Transfusion events and risks of viral or bacterial contamination are more frequent with platelet infusions (11‰) than with erythrocyte (3.5‰) or FFP (0.8‰) infusions [14]. The risk of TRALI (transfusion-related acute lung injury) is also higher: on average 1:2,000 for platelets, while it is 1:5,000 for packed red cells [16]. A febrile or hypotensive episode is common during infusion (see Chapter 28 Blood product risks). Mortality rate is 1:40,000 transfusions.
Platelet administration is only justified in haemorrhagic procedures performed in patients with either dysfunctional or insufficient thrombocytes: patients on uninterrupted antiplatelet therapy and patients with bone marrow failure or acute consumption. Indeed, platelet transfusion in general surgery is associated with an increased risk of stroke and arterial thrombosis (OR 1.55) and excess mortality (OR 2.40) [6]. The same association is found in cardiac surgery, with excess stroke (OR 2.56) and mortality (OR 4.76) in thrombo-transfused patients [15]. In neurology, platelet transfusion doubles the risk of death and complications after haemorrhagic stroke in patients previously on antiplatelets (OR 2.05) [3].On the other hand, normalising platelet function in patients on antiplatelets puts them at increased risk of vascular thrombosis, particularly in stents and endoprosthesis. It is therefore clear that prophylactic administration of thrombocytes is more dangerous than beneficial and is only recommended in cases of bone marrow failure or equivalent.
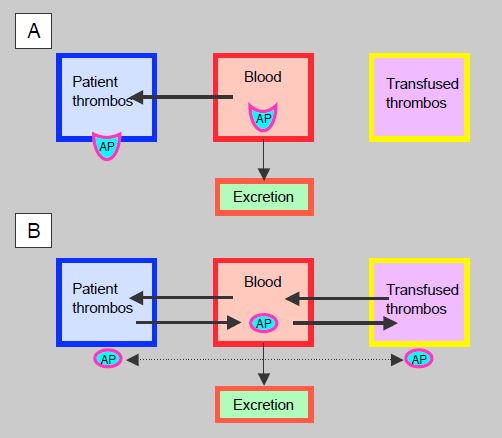
Under antiplatelet therapy with an irreversible agent (aspirin, clopidogrel, prasugrel), platelets are inhibited for their entire life span, but the substance is permanently bound to thrombocytes. As soon as equilibrium is reached between plasma and platelet receptors, thrombocyte aggregability is no longer dependent on the serum level of the agent. When the serum level decreases with elimination (12.5% after 3 half-lives), freshly circulated platelets or transfused platelets function normally, which is the case 12 hours after aspirin or prasugrel ingestion and 24 hours after clopidogrel ingestion, even though the patient's thrombocytes are still blocked for several days. The situation is different with reversible antiplatelet agents such as ticagrelor, because the substance is in constant equilibrium between plasma and receptors, whether the platelets are the patient's own or those from a transfusion (Figure 8.23). In this case, inhibition of aggregability is directly proportional to the serum level for all platelets, and the transfusion will only be effective after at least 3 half-lives, in this case beyond 36 hours. As ticagrelor has a high affinity and strong binding to ADP receptors, back-diffusion from platelets is slow, so the clinical effect tends to be extended beyond the theoretical pharmacokinetic time. Despite having a lower risk of spontaneous bleeding than clopidogrel or prasugrel, ticagrelor raises a serious problem when bleeding requires platelet transfusion, as this will be less effective for 2-3 days after the last dose [8]. An in vitro study has shown that addition of platelets to blood samples from patients on anti-platelet drugs suppresses the effect of aspirin, significantly reduces the effect of clopidogrel, but is less effective in reversing the effect of ticagrelor [5].
Figure 8.23: Antiplatelet agents (AP) and thrombocyte transfusion. A: Irreversible agents such as clopidogrel or prasugrel diffuse from the blood to the platelets and remain bound there while the serum level decreases according to the plasma half-life. The degree of platelet inhibition does not depend on the serum level of the substance. Transfused platelets encounter less circulating agent the longer the time since last administration: 50% after1 half-life, 25% after2 half-lives and 12.5% after3 half-lives; they function normally beyond 2-3 half-lives, while the patient's platelets remain permanently blocked. B: With reversible agents such as ticagrelor, a dynamic equilibrium is established between the blood, the patient's platelets and the transfused platelets; the substance is distributed according to concentration gradients. The degree of platelet inhibition depends directly on the serum level of the substance. Since ticagrelor is strongly bound to ADP receptors, back-diffusion to the plasma is slowed, but the substance can diffuse between platelets (cross-diffusion), which also impairs the function of transfused thrombocytes.
Transfusion of platelet concentrates during cardiac procedures should be restricted to the following conditions
- Thrombocytopenia < 50,000/mcL ;
- Platelet dysfunction proven by functional test (Multiplate™, VerifyNow™, etc);
- Hemorrhage on uninterrupted preoperative antiplatelet therapy;
- Hemorrhage not controlled by usual measures.
Thromboplegia
By blocking platelet aggregation induced by the action of preformed IgG antibodies on PF4-heparin complexes during ECC with a short-acting antiplatelet agent, the stimulation and consumption of thrombocytes by contact with foreign surfaces, which leads to postoperative thrombocytopenia and platelet dysfunction, should be avoided. This thromboplegia, or thromboanesthesia, is an option already used in patients with anti-heparin antibodies (see HIT). Several possibilities are considered.
- Heparin (usual dose) + prostacyclin (Iloprost® ): increases intraplatelet cAMP and inhibits activation. Half-life 15-30 min. Infusion of 3-12 ng/kg/min (avg 7.6 ng/kg/min), HIPA (Heparin-induced platelet aggregation) assay for activation < 5%. The infusion is halved after protamine and reduced to one quarter on arrival in the ICU and stopped 1 hour later [10]. Disadvantages: iloprost causes hypotension (regulated by norepinephrine), and intraoperative HIPA tests are time consuming (30-40 minutes).
- Heparin (bolus 5,000 IU and infusion 1,000 IU/h) + tirofiban (Aggrastat® ): blocker of the GP IIb/IIIa receptor of platelets, responsible for binding of platelets with fibrinogen (see Figures 8.12 and 8.13). Half-life: 2 hours. Bolus 0.4 mcg/kg, then infuse 0.15 mcg/kg/hour. Stop 1 hour before the end of the ECC [7].
- Cangrelor (Kengrexal® ): a reversible direct P2Y receptor inhibitor12 , marketed only for PCI and acute coronary syndrome. Half-life 9 minutes. Infusion 0.75 mcg/kg/min [1]. Interrupted 30 minutes before protamine. It blocks platelet activation induced by ECC and hypothermia, and reduces the complications associated with them in vitro and in vivo [9].
Desmopressin
Desmopressin (DDAVP: deamino-D-arginine vasopressin, Octostim® , Minirin® ) stimulates the production of factor VIII and von Willebrand factor by the endothelium; it increases their levels by 3 to 5 times and improves platelet adhesion to collagen. It is therapeutic in certain conditions with specific thrombocyte dysfunction (non-severe haemophilia A, congenital or acquired von Willebrand disease type I, uraemia, liver disease, severe aortic stenosis) (see Chapter 21, Haematological Diseases). Although responsiveness of platelets in vitro to various aggregation stimulants is markedly improved by DDAVP [13], reduction in bleeding is modest in clinical practice [18]. Desmopressin is useful in platelet dysfunction, reducing blood loss by 23% and transfusions by 25% during coronary revascularisation in ECC in patients receiving antiplatelet therapy; the rate of re-intervention for haemostasis is reduced (OR 0.39) [4,12]. Its dosage is 0.3 mcg/kg intravenously over 30 minutes (half-life 4-5 hours). Simultaneous administration of tranexamic acid is recommended as desmopressin also stimulates fibrinolysis.
| Platelet transfusion |
|
Intraoperative indications for active bleeding :
- Thrombocytopenia < 50,000/mcL
- Thrombocyte dysfunction proven by a platelet aggregability test
- Bleeding on uninterrupted antiplatelet therapy
- Hemorrhage uncontrollable by usual measures
Risk of bacterial or viral contamination (1.1%) is much higher than with blood or FFP transfusions. Platelet overtransfusion carries a definite risk of vascular thrombosis (heart attack, stroke). Recommendation: no prophylactic platelet transfusion outside of bone marrow aplasia.
Desmopressin: improves platelet aggregability, stimulates production of factor VIII and von Willebrand factor by the endothelium. Potentially useful in bleeding on antiplatelets. Dosage: 0.3 mcg/kg.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- Angiolillo DJ, FIRSTENBERG MS, PRICE MJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery. JAMA 2012; 307:265-74
- ANTONIOU T, KAPETANAKIS EI, THEODORAKI K, et al. Cardiac surgery in patients with heparin-induced thrombocytopenia using preoperatively determined dosages of Iloprost. Heart Surg Forum 2002; 5:354-7
- BAHAROGLU MI, CORDONNIER C, SALMAN RS, et al. Platelet transfusion versus standard careafteracute stroke due to spontaneous cerebral hemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016; 387:2605-13
- DESBOROUGH MJR, OAKLAND KA, LANDONI G, et al. Desmopressin for treatment of platelet dysfunction and reversal of antiplatelet agents: a systematic review and meta-analysis of randomized controlled trials. J Thromb Haemost 2017; 15:263-72
- HANSSON EC, HAKIMI CS, ARSTRÖM-OLSSON K, et al. Effects of ex vivo platelet supplementation on platelet aggregability in blood samples from patients treated with acetylsalicylic acid, clopidogrel, or ticagrelor. Br J Anaesth 2013; 112:570-5
- KHORANA AA; FRANCIS CW, BLUMBERG N, et al. Blood transfusions, thrombosis and mortality in hospitalized patients with cancer. Arch Intern Med 2008; 168: 2377-81
- KOSTER A, KUKUCKA M, BACH F, et al. Anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II and renal impairment using heparin and the platelet glycoprotein IIb/IIIa antagonist tirofiban. Anesthesiology 2001; 94:245-51
- KOZEK-LANGENECKER SA, AHMED AB, AFSHARI A, ALBALADEJO P, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. First update 2016. Eur J Anaesthesiol 2017; 34: 332-95
- KRAJEWSKI S, KURZ J, NEUMANN B, et al. Short-acting P2Y12 blockade to reduce platelet dysfunction and coagulopathy during experimental extracorporeal circulation and hypothermia. Br J Anaesth 2012; 108:912-21
- PALATIANOS G, MICHALIS A, ALIVIZATOS P, et al. Perioperative use of iloprost in cardiac surgery patients diagnosed with heparin-induced thrombocytopenia-reactive antibodies or with true HIT (HIT-reactive antibodies plus thrombocytopenia): an 11-year experience. Am J Hematol 2015; 90:608-17
- PEYROU V, LORMEAU JC, HERAULT JP, et al. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost 1999; 81: 400-6
- RANUCCI M, NANO G, PAZZAGLIA A, et al. Platelet mapping and desmopressin reversal of platelet inhibition during emergency carotid endarterectomy. J Cardiothorac Vasc Anesth 2007; 21: 851-4
- REITER RA, MAYR F, BLAZICEK H, et al. Desmopressin antagonizes the in vitre platelet dysfunction induced by GPIIb/IIIa inhibitors and aspirin. Blood 2003; 102:4594-9
- SPIESS BD. Platelet transfusions: the science behind safety, risks and appropriate applications. Best Pract Res Clin Anaesthesiol 2010; 24: 65-83
- SPIESS BD, ROYSTON D, LEVY JH, et al. Platelet transfusions during coronary artery bypass graTF surgery are associated with serious adverse outcomes. Transfusion 2004; 44: 1143-8
- TRIULZI DJ. Transfusion-related acute lung injury: Current concepts for the clinician. Anesth Analg 2009; 108:70-6
- UIJTTEWAAL WS, NIJHOF EJ, BRONKHORST PJ, et al. Near-wall excess of platelets induced by lateral migration of erythrocytes in flowing blood. Am J Physiol 1993; 264: H1239-44
- WEBER CF, DIETRICH W, SPANNAGL M, et al. A point-of-care assessment of the effects of desmopressin on impaired platelet function using Multiple Electrode whole-blood Aggregometry in patientsaftercardiac surgery. Anesth Analg 2010; 110:702-7