Tricuspid valve
Tricuspid insufficiency (TI) is generally caused by dilation of the tricuspid annulus secondary to volume overload and RV enlargement. It is often the factor that triggers right ventricular failure as it leads to a vicious circle of mutually increasing regurgitation and dilation. Surgical repair is justified to end this process in cases of moderate to severe TI. In rarer cases, TI is caused by dysplasia and congenital valve prolapse. This may be massive in case of Ebstein's anomaly.
Mitral valve
The mitral valve is prone to a huge range of pathologies in children as in adults. The most common congenital anomalies are prolapse and isolated cleft, which cause regurgitation, and double-orifice mitral valve or parachute mitral valve (2 leaflets and a single papillary muscle), which may be stenotic or insufficient. Parachute valve is generally associated with Shone's syndrome, which combines 4 left-sided stenosing lesions: parachute mitral valve (functional restriction), supravalvular mitral annulus, subaortic stenosis, and coarctation of the aorta (see Anomalies of the Left Ejection Pathway). Indication for surgery is dependent on impact on the LV (dilation, failure), pulmonary post-capillary hypertension (post-capillary PHT), arrhythmias, and clinical signs (dyspnoea) [1]. In cases of stenosis, the indication is a mean gradient > 12 mmHg. Pulmonary vasodilators (NO, prostaglandins, etc.) are contraindicated in post-capillary PHT, since the increased pulmonary flow increases stasis and the risk of acute pulmonary edema. When treating children, the aim is evidently to perform surgical repair rather than valve replacement with mechanical prostheses, as these are too large for young children. Moreover, they require permanent anticoagulation and must be changed during growth [2].
A rare malformation known as cor triatriatum, which involves median septation of the LA may be revealed by symptoms reminiscent of mitral stenosis. A transverse membrane perforated by an orifice of varying size splits the left atrium into two separate chambers. The pulmonary veins join up LA upstream of the membrane into a posterior chamber. The atrial appendage opens into an anterior chamber, which leads to the mitral valve (Figure 14.42). Symptoms depend on the size of the orifice in the membrane. Surgery involves resecting the membrane under CPB. This malformation is managed in the same way as mitral stenosis.
Whatever the etiology, the management of anaesthesia in case of mitral stenosis implies some specific constraints (see Chapter 11 - Mitral Stenosis).
Tricuspid insufficiency (TI) is generally caused by dilation of the tricuspid annulus secondary to volume overload and RV enlargement. It is often the factor that triggers right ventricular failure as it leads to a vicious circle of mutually increasing regurgitation and dilation. Surgical repair is justified to end this process in cases of moderate to severe TI. In rarer cases, TI is caused by dysplasia and congenital valve prolapse. This may be massive in case of Ebstein's anomaly.
Mitral valve
The mitral valve is prone to a huge range of pathologies in children as in adults. The most common congenital anomalies are prolapse and isolated cleft, which cause regurgitation, and double-orifice mitral valve or parachute mitral valve (2 leaflets and a single papillary muscle), which may be stenotic or insufficient. Parachute valve is generally associated with Shone's syndrome, which combines 4 left-sided stenosing lesions: parachute mitral valve (functional restriction), supravalvular mitral annulus, subaortic stenosis, and coarctation of the aorta (see Anomalies of the Left Ejection Pathway). Indication for surgery is dependent on impact on the LV (dilation, failure), pulmonary post-capillary hypertension (post-capillary PHT), arrhythmias, and clinical signs (dyspnoea) [1]. In cases of stenosis, the indication is a mean gradient > 12 mmHg. Pulmonary vasodilators (NO, prostaglandins, etc.) are contraindicated in post-capillary PHT, since the increased pulmonary flow increases stasis and the risk of acute pulmonary edema. When treating children, the aim is evidently to perform surgical repair rather than valve replacement with mechanical prostheses, as these are too large for young children. Moreover, they require permanent anticoagulation and must be changed during growth [2].
A rare malformation known as cor triatriatum, which involves median septation of the LA may be revealed by symptoms reminiscent of mitral stenosis. A transverse membrane perforated by an orifice of varying size splits the left atrium into two separate chambers. The pulmonary veins join up LA upstream of the membrane into a posterior chamber. The atrial appendage opens into an anterior chamber, which leads to the mitral valve (Figure 14.42). Symptoms depend on the size of the orifice in the membrane. Surgery involves resecting the membrane under CPB. This malformation is managed in the same way as mitral stenosis.
Whatever the etiology, the management of anaesthesia in case of mitral stenosis implies some specific constraints (see Chapter 11 - Mitral Stenosis).
- Normal preload;
- Systemic vasoconstriction;
- Slow heart rate;
- Positive-pressure ventilation well tolerated;
- Required haemodynamics: normovolaemic - slow - vasoconstricted.
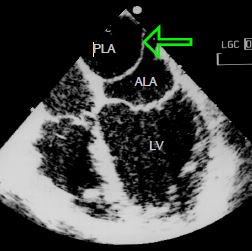
Figure 14.42: Cor triatriatum. A membrane (arrow) separates the LA into two chambers, one of which is posterior (PLA) and receives the pulmonary veins; the other is anterior (ALA) and is connected to the atrial appendage and mitral valve. This membrane is positioned at the level of the fossa ovalis membrane on the septum and at the level of the crest between the left superior pulmonary vein and the left atrial appendage.
Anaesthesia for children with mitral insufficiency (MI) is guided by the same principles as for adults (see Chapter 11 - Mitral Insufficiency).
- Normal preload;
- Systemic vasodilation (MI ↓ if SVR ↓);
- Normal or moderately elevated heart rate;
- Positive-pressure ventilation well tolerated;
- Inotropic stimulation without alpha effect (LV dysfunction is concealed by low afterload due to systolic regurgitation in the LA);
- Required haemodynamics: normovolaemic - tonic - vasodilated.
Following repair or replacement of the mitral valve, the post-CPB situation differs depending on whether the basic disorder is stenosis or insufficiency.
- Stenosis: residual gradient < 5 mmHg. The LV is small due to the chronic filling restriction imposed on it. After CPB, it is in a congestive situation since filling is normal but its end-diastolic volume remains low. Through tachycardia, it is able to increase its flow by fractionating the stroke volume; inotropic support is useful.
- Insufficiency: while the LV benefited from low afterload due to the MI, it must expel blood against systemic impedance after CPB, which constitutes an elevation of its afterload. Inotropic support without vasoconstriction is required (dobutamine, milrinone).
- Pulmonary hypertension: the post-capillary component of PHT is normalised, but not the pre-capillary component – it may be necessary to lower PVR (NO, prostaglandin, milrinone).
- Lateral ischaemia (TEE): a lesion of the circumflex artery is possible as its path adjoins the posterior mitral annulus.
| Congenital mitral valve anomalies |
|
Double-orifice or parachute mitral valve disease. Management:
- Predominant insufficiency: normovolaemia, low SVR, inotropic support (normovolaemic - tonic - vasodilated) - Predominant stenosis: normovolaemia, slow HR, raised SVR (normovolaemic - slow - vasoconstricted) Cor triatriatum: transverse membrane across the LA with an orifice of variable size, reminiscent of mitral stenosis. Management: normovolaemic patient, slow heart rate, systemic vasoconstriction. |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- SCHAVERIEN MV, FREEDOM RM, McCRINDLE BW. Independent factors associated with outcomes of parachute mitral valve in 84 patients. Circulation 2004; 103:2309-13
- STELLIN G, PADALINO MA; VIDA VL, et al. Surgical repair of congenital mitral valve malformation in infancy and childhood: a single-center 36-year experience. J Thorac Cardiovasc Surg 2010; 140:1238-44
