Aortic valve stenosis is the most common form of valvular heart disease in adults, with a prevalence of approximately 5% over the age of 65 [3,5,6,10]. There are several causes of aortic stenosis.
- The most common cause is calcific degeneration of a normal three-cusp valve, usually seen in patients over the age of 65. Calcific deposits in the form of nodular growths without commissural fusion stiffen the valves and block them in a semi-closed position. This valvular calcification is often associated with calcification of the mitral annulus and systemic arterial atheromatosis, in patients with hypercholesterolaemia, arterial hypertension, diabetes and smoking; the presence of inflammatory processes in the calcifying lesions indicates the relationship between aortic stenosis and atherosclerosis (Figure 11.100 and Figure 11.101) [13].
Video: Tight aortic stenosis in 50° short-axis view; the valve is tricuspid and heavily calcified.
Video: Long-axis three-dimensional view of a tight aortic stenosis.
- Congenital aortic bicuspidism (prevalence: 1-2% of the population, of whom 2% develop stenosis) is characterised by an ellipsoidal orifice and two cusps of unequal size, the larger of which often contains a median raphe (Figure 11.102) [6].
Video: Three-dimensional short-axis view of aortic bicuspidism.
Video: Three-dimensional short-axis view ("en face" view of the aorta) of tight aortic stenosis on bicuspid artery.
- The lesion secondary to ARF is currently rare in developed countries (<10% of cases). It is characterised by commissural fusion, fibrosis and neovascularisation of the cusps and shows a mildly calcified thickening of the leaflets, predominantly at the commissures (see Figure 11.100B); it is often associated with mitral disease of the same origin. It progresses more slowly than calcific degeneration.
- Aortic sclerosis is an irregular thickening of the leaflets without stenosis or increased pressure gradient; it occurs in 25% of people aged > 65 years. It is associated with arterial hypertension, hypercholesterolaemia, diabetes, smoking and an increased risk of myocardial infarction [12].
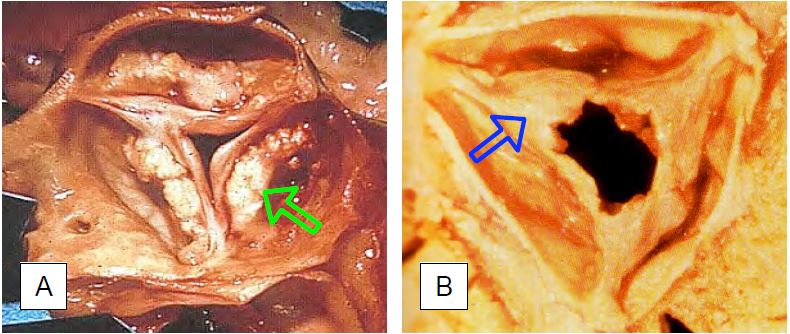
Figure 11.100: Pathological images of acquired aortic stenosis of the tricuspid aortic valve. A: Calcified degeneration in the elderly; calcifications are located in the body of the leaflets. B: Stenosis after ARF; calcifications are less significant and are located in the fused commissures: Braunwald E. Valvular heart disease. In: Braunwald E. ed. Heart Disease. Philadelphia, WB Saunders Co, 1997, 1007-76].
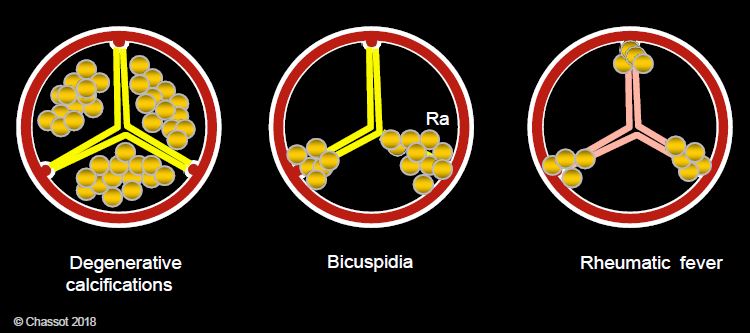
Figure 11.101: Pathological appearance of calcifications in aortic stenosis. Calcifications are located in the body of the cusps in degeneration, on the raphe (Ra) and at the commissures in bicuspidosis, and at the 3 commissures in ARF.
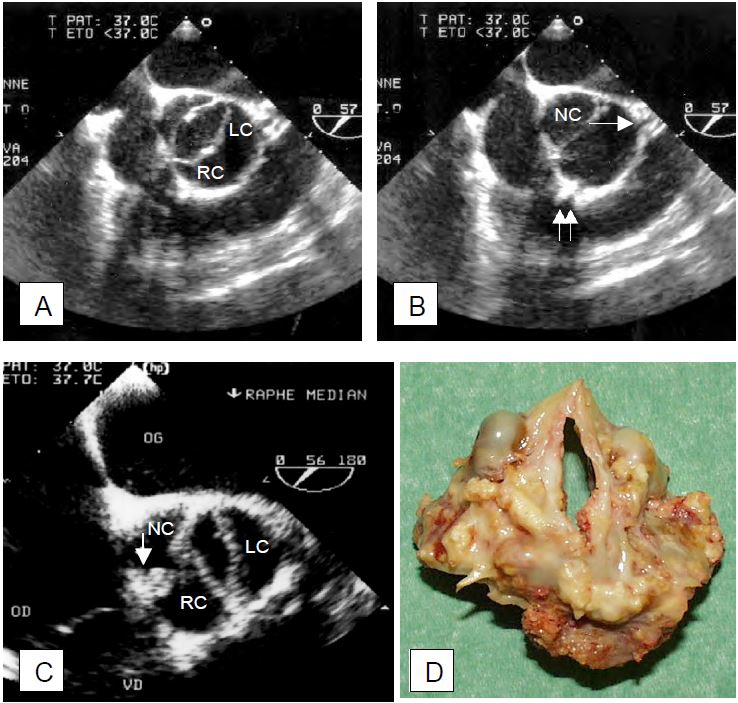
Figure 11.102: Bicuspid aortic valve. A: Transesophageal echocardiogram in systole; the valve orifice is elliptical; the right (R) and left (L) coronary leaflets are fused. B: image in diastole; the beginning of the common trunk (→) and the right coronary artery (↑↑) are visible; NC: non-coronary leaflet. C: The arrow indicates the presence of a raphe at the site of fusion between the right coronary and non-coronary leaflets; the remaining orifice is elliptical, banana-shaped (vertical in the short-axis image of the valve). D: Surgical specimen from the case shown in C.
Under the age of 65, the usual distribution of aetiologies is: bicuspidosis 50%, ARF 25% and degeneration 18%. Over the age of 70, the distribution changes: degeneration 48%, bicuspidosis 27%, ARF 23% [5]. Stenosis of the bicuspid valve becomes clinically symptomatic about 20 years earlier than stenosis of the tricuspid valve. Traditionally, two forms of bicuspidosis are distinguished anatomopathologically (Figure 11.103).
- True bicuspidosis: there are only 2 embryonic cusp buds; the two valve leaflets are of variable size and proportion, generally slightly unequal. The opening slit is oval and its orientation is right/left or anterior/posterior.
Video: Short-axis view of "true" bicuspidism in a young adult; there are only two cusps in the anteroposterior position; the systolic opening is incomplete.
Video: Systolic flow through a bicuspid aortic valve; the opening takes on an oval shape between the right and left cusps.
- Acquired bicuspidism: as there are originally 3 buds, two of which later fuse, 3 commissures are found in their normal position, but one cusp is twice the size of the other; it usually has a fibrous raphe in the middle marking the position of the fused commissure. The orifice is banana shaped. Bicuspidism due to secondary calcification falls into this category.
Video: Calcified and stenotic bicuspid aortic valve; the opening is narrowed; it occurs between the non-coronary cusp and the right and left coronary cusps, which are fused (acquired bicuspidism). 3 commissures are clearly visible.
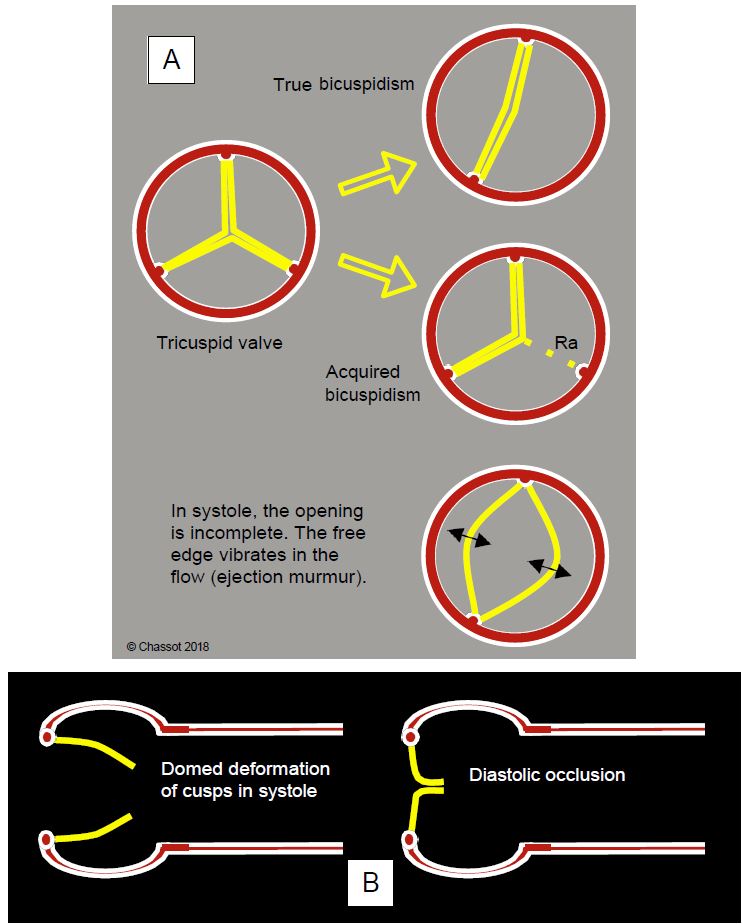
Figure 11.103: Types of bicuspidism. In true bicuspidism there are embryologically only two valve buds and two commissures; the position of the latter is variable, as is the size of each cusp. In acquired bicuspidism, the valve started in the embryo with three leaflets and three commissures, equidistantly positioned in an equilateral triangle; secondarily, two of these leaflets fused, leaving only a trace of the first commissure in the form of a raphe (Ra). Since the diameter of a circle is less than half its circumference, the free edges of the cusps cannot be correctly aligned parallel to the flow when the valve is open in systole; they create a relative stenosis and vibrate in the flow, causing a systolic murmur unrelated to the stenosis, since their opening surface is still normal. B: Aortic bicuspidity; systolic doming of the leaflets; the free edges cannot be positioned normally in relation to the aortic wall.
This distribution has now been replaced by a classification based on the number of raphe: none, one or two (see Aortic bicuspidity) [14].
Although anatomically quite distinct, the different aetiologies of aortic stenosis are often difficult to distinguish at an advanced stage of the disease. It is not uncommon for the described anatomical deformities to prevent the valve from sealing in diastole, and associated aortic insufficiency (AI) is common but haemodynamically insignificant.
Video: Aortic stenosis in long-axis view; the stenosis is tight, systolic flow is accelerated and narrow in the ascending aorta; presence of a small diastolic insufficiency.
Congenital cases may include unicuspid valves and supra- or subvalvular stenosis. The obstruction can sometimes be located below the valve, in the LV pressure chamber (LVOT). There are two categories of subaortic stenosis.
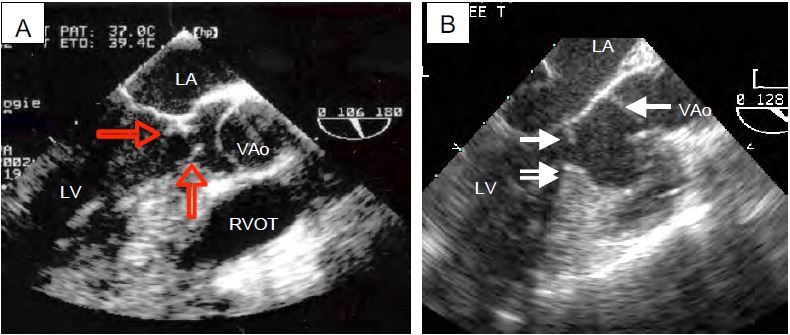
- Fixed stenosis: subaortic fibromuscular membrane partially obstructing the LVOT; this is a congenital lesion associated with hypotrophy of the aortic valve (Figure 11.104).
Video: Fixed subaortic stenosis in the form of a fibrous crescent in the chasse chamber, seen on the septal side of the LVOT.
- Dynamic muscle stenosis: caused by obstructive cardiomyopathy (HOCM) or concentric hypertrophy in a particular constellation of hypovolaemia, inotropic excess and low afterload (see Dynamic subaortic stenosis).
Fig 11.104: Congenital subaortic stenosis. A: A circular membrane (arrows) narrows the outflow tract anterior to the aortic valve; it is attached to the interventricular septum, the anterior leaflet of the mitral valve and the posterior wall of the LV. B: Combination of a subaortic membrane (white arrow) and a muscular septal spur (double arrow). The aortic valve (VAo) is often hypotrophic downstream of the infundibular stenosis. RVOT: right ventricular cavity.
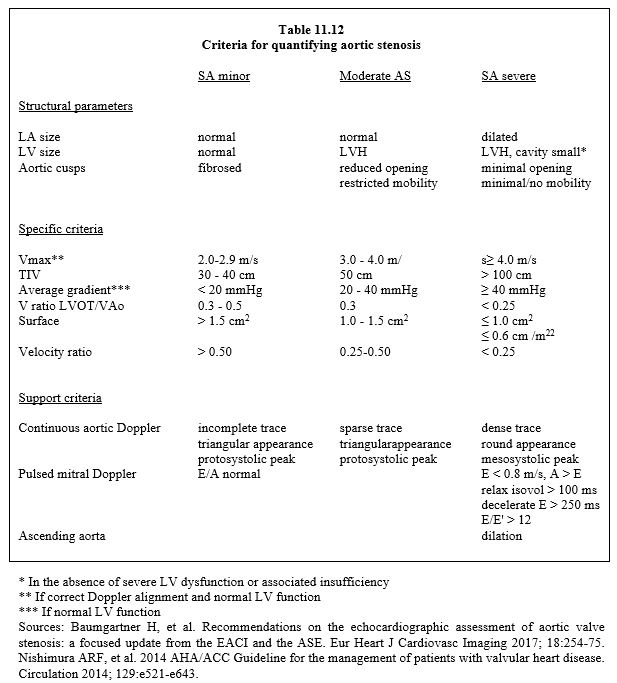
The normal aortic valve area is 2.5 - 4.0 cm2 (index: 2 cm2/m2). Stenosis is quantified in three degrees (Table 11.12) [2,9,15].
- Mild stenosis: Surface 1.5 cm2 ΔPmean < 20 mmHg Vmax < 3 m/s
- Moderate stenosis: Surface 1.0 - 1.5 cm2 ΔPmean 20-40 mmHg Vmax 3 - 4 m/s
- Severe stenosis: Surface ≤ 1.0 cm2 ΔPmean ≥ 40 mmHg Vmax ≥ 4 m/s
Sindexed ≤ 0.6 cm2/m2
- Aortic surface area ≤ 1.0 cm2 (≤ 0.6 cm2/m2)
- Mean pressure gradient (ΔP) ≥ 40 mmHg ( ΔPmax ≥ 100 mmHg);
- Aortic flow velocity ≥ 4.0 m/s;
- Ratio Vmax LVOT / Vmax VAo < 0.25;
- Integral velocity (VTI) > 100 cm;
- Concentric hypertrophy of the LV (posterior wall thickness in diastole > 1.2 cm), LV cavity reduced in size;
- Preserved systolic function;
- Diastolic dysfunction with LA enlargement.
- In tight aortic stenosis, the pressure gradient increases under dobutamine (increase of > 20 mmHg), but not the valve surface, which remains fixed due to calcification. The ratio between the velocity in the LVOT (VTILVOT ) and the velocity across the aortic valve (VTIVAo ) is < 0.25 in narrow aortic stenosis (normal: > 0.8). With dobutamine, this ratio increases further in tight stenosis because VTIVAo increases more than VTILVOT.
- In cardiomyopathy, however, the measured valve area increases because the functional improvement in the LV allows the stroke volume to increase and the valve to open further, but the gradient does not change [7,9]. In the presence of cardiomyopathy and functional stenosis, the ratio of VTILVOT / VTIVao increases because the acceleration is much greater in the LVOT than through the valve, which increases its opening and adapts to the increase in stroke volume without changing its gradient [4].
| Aortic valve stenosis |
| Three aetiologies: calcific degeneration (most common > 65 years of age), bicuspidosis (half of cases < 60 years of age), ARF (rarer in developed countries).
Definition of degrees of stenosis (preserved LV function):
- Mild superficial stenosis 1.5 cm2 ΔPmean < 20 mmHg
- Moderate stenosis 1.0 - 1.5 cm2 ΔPmean 20 - 40 mmHg
- Tight stenosis Surface area ≤ 1.0 cm2 (0.6 cm2/m2) ΔPmean ≥ 40 mmHg
Tight stenosis is also characterised by
- Vmax aortic flow ≥ 4 m/s
- Integral Aortic Velocity (VTIVAo ) > 100 cm
- VTI ratio VTILVOT / VTIVao < 0.25
- Concentric LVH (preserved systolic function but diastolic dysfunction)
|
References
- ADDA J, MIELOT C, GIORGI R, et al. Low flow/low gradient severe aortic stenosis despite normal ejection fraction is associated with severe left ventricular dysfunction as assessed by speckle-tracking echocardiography: a multicenter study. Circ Cardiovasc Imaging 2012; 5:27-35
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- DARE AJ, VEINOT JP, EDWARDS WD, et al. New observations on the etiology of aortic valve disease. Hum Pathol 1993; 24:1330
- DEFILIPPI CR, WILLETT DL, BRICKNER ME, et al. Usefullness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. Am J Cardiol 1995; 75:191-4
- FOX CS, VASAN RS, PARISE H, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation 2003; 107:1492-6
- IUNG B, BARON G, BUTCHART EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J 2003; 24:1231-43
- LASKEY WK, KUSSMAUL WG, NOORDEGRAAF A. Systemic arterial response to exercise in patients with aortic valve stenosis. Circulation 2009, 119:996-1004
- MINNERS J, ALLGEIER M, GOHLKE-BAERWOLF C, et al. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010; 96:1463-8
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- NKOMO VT, GARDIN JM, SKELTON TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005-11
- OTTO CM, BURWASH IG, LEGGET ME, et al. Prospective study of asymptomatic valvular aortic stenosis: Clinical, echocardiographic and exercise predictors of outcome. Circulation 1997; 95:2262-70
- OTTO CM, LIND BK, KITZMAN DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999; 341:142-7
- PELETIER M, TROJETTE F, ENRIQUEZ-SARANO M, et al. Relation between cardiovascular risk factors and nonrheumatic severe calcific aortic stenosis among patients with a three-cuspid aortic valve. Am J Cardiol 2003; 91:97-105
- SIEVERS HH, SCHMIDTKE C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133:1228-33
- VAHANIAN A, ALFIERI O, ANDREOTTI F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012; 33:2451-96