No single solution can be applied to all children. Several techniques are possible using a main agent and add-on agents. These should be chosen in light of cardiac pathology, age, CPB duration, level of hypothermia, and postoperative forecasts [6]. In cases of children aged over 3 months with a simple form of heart disease and brief CPB (< 90 minutes) without deep hypothermia (T° > 34°C), it is desirable to extubate the child early (fast-track protocol). In such instances, several combinations may be used:
- Fentanyl (10-25 mcg/kg) or sufentanil (0.5-2.5 mcg/kg) combined with a volatile agent at 1-1.5 MAC (sevoflurane or isoflurane);
- Fentanyl (10-25 mcg/kg) or sufentanil (0.5-2.5 mcg/kg) combined with a propofol infusion (10-20 mg/kg/hour);
- Remifentanil (0.1-0.2 mcg/kg/min) and isoflurane, sevoflurane or propofol infusion (2-10 mg/kg/hour)
Based on current data, propofol infusions cannot be recommended for young children. In children aged under two years, the choice is restricted to a combination of fentanyl-halogenated agent, fentanyl-ketamine, or fentanyl-midazolam. Postoperative analgesia may be provided by a continuous infusion of morphine (20 mcg/kg/hour) or midazolam-sufentanil.
In any complex cases with prolonged intubation (infants or neonates, cyanotic heart disease, pulmonary hypertension, ventricular failure, CPB > 90 min, deep hypothermia), anaesthesia should ideally be based on the use of midazolam (0.05-0.1 mg/kg IV) and fentanyl or sufentanil at doses inhibiting the stress response (approximately 50-100 mcg/kg and 5-10 mcg/kg respectively). These doses are split between induction, sternotomy, CPB and sternum closure. Anaesthesia is maintained by adding sevoflurane (≤ 1 MAC), isoflurane (≤ 1 MAC if reduced SVR is advantageous), midazolam (0.1 mg/kg/h, sympathicolysis), ketamine (1 mg/kg/h, sympathetic stimulation) or propofol (2-10 mg/kg/h, reduced preload) depending on the required haemodynamic impact. Sevoflurane and fentanyl offer the greatest haemodynamic stability in very young children. A muscle relaxant (generally vecuronium or rocuronium) is chosen based on whether heart rate and arterial pressure must be maintained and on the required duration of action. The vagolytic effect of pancuronium bromide can be useful for limiting opiate-related bradycardia, which is poorly tolerated by young children.
Induction
Three induction methods are recommended depending on the child’s condition:
In any complex cases with prolonged intubation (infants or neonates, cyanotic heart disease, pulmonary hypertension, ventricular failure, CPB > 90 min, deep hypothermia), anaesthesia should ideally be based on the use of midazolam (0.05-0.1 mg/kg IV) and fentanyl or sufentanil at doses inhibiting the stress response (approximately 50-100 mcg/kg and 5-10 mcg/kg respectively). These doses are split between induction, sternotomy, CPB and sternum closure. Anaesthesia is maintained by adding sevoflurane (≤ 1 MAC), isoflurane (≤ 1 MAC if reduced SVR is advantageous), midazolam (0.1 mg/kg/h, sympathicolysis), ketamine (1 mg/kg/h, sympathetic stimulation) or propofol (2-10 mg/kg/h, reduced preload) depending on the required haemodynamic impact. Sevoflurane and fentanyl offer the greatest haemodynamic stability in very young children. A muscle relaxant (generally vecuronium or rocuronium) is chosen based on whether heart rate and arterial pressure must be maintained and on the required duration of action. The vagolytic effect of pancuronium bromide can be useful for limiting opiate-related bradycardia, which is poorly tolerated by young children.
Induction
Three induction methods are recommended depending on the child’s condition:
- Children whose haemodynamic state enables them to withstand induction with volatile agents: mask induction with sevoflurane is possible. Once the venous line is in place, the halogenated agent is discontinued and intravenous anaesthesia initiated: fentanyl, midazolam, propofol or ketamine depending on haemodynamic constraints.
- Children who are calm and drowsy due to premedication: fitting a venous line for induction is feasible without causing any excessive reactions.
- Children who are exhausted by their heart disease or already have a venous line fitted – induction is performed intravenously using fentanyl combined with midazolam or etomidate as required.
In the event of bradycardia, atropine (0.01 mg/kg IV – minimum dose 0.1 mg) is recommended before manipulating the airways of children aged up to two years.
Intubation
Young children’s pulmonary functional residual capacity (FRC) and oxygen reserve are reduced while metabolic requirements are high. The apnoea period imposed by the intubation procedure should be kept as brief as possible and preceded by a period of hyperventilation using a mask. Moreover, it is essential to reduce PaCO2 in the event of pulmonary hypertension. However, high intrathoracic pressure (forced positive-pressure ventilation, Valsalva manoeuvre) forces a bidirectional shunt in the R-to-L direction (PRA > PLA). Children with predominantly L-to-R ASDs may suddenly desaturate or embolise at arterial level when ventilated at an excessive positive pressure. Since hyperventilation renews the alveolar air more quickly than the pulmonary blood flow, the alveolar concentration of anaesthetic gas is increased and therefore its blood concentration also rises. This induction-related acceleration is more pronounced for soluble agents (halothane) than for agents with low solubility (desflurane) – isoflurane and sevoflurane are middle-ranking in this respect [31].
Nasal intubation is preferable for children weighing up to 5 kg or in cases of prolonged intubation for children aged up to 2 years. Given the occasional need to institute hyperventilation and due to low pulmonary compliance, an internal tube diameter that prevents leakage below a pressure of 20 cm H2O is required. A cuffed tube ensures air-tightness and is recommended in all cases, except for neonates [2]. In 25% of cases, cardiac malformations are combined with other malformations. Some subjects also have facial dysmorphism, which makes intubation difficult. Anaesthetists must plan for this in the preoperative examination to avoid being unexpectedly faced with a cyanotic, curarised child who is difficult to ventilate and moreover has an invisible glottis!
High-volume, rapid administration of fentanyl often prompts rigidity and choreoathetotic movements through extrapyramidal stimulation. Mask ventilation without curare is therefore difficult or even impossible. Bradycardia reduces cardiac output. This situation can rapidly become dangerous. The best solution is rapid muscle relaxation with 0.3 mg/kg of vecuronium or 1.2 mg/kg of rocuronium (time required until intubation is possible: 35 seconds), or possibly 1-2 mg/kg of succinylcholine (time to intubation: < 15 seconds) [28].
Ventilation
The reduction in pulmonary compliance and increase in airway resistance in children with L-to-R shunting and increased pulmonary blood flow is proportionate to vascular overload. The correlation with PAP is less evident [29]. The pulmonary vessels may compress bronchi and restrict flow due to their excessive size.
Positive-pressure ventilation negatively impacts the right heart and has a positive effect on the left heart. PVR is minimal if the pulmonary volume matches the functional residual capacity (see Figure 14.19) [9]. Hypoxia and hypercapnia lower local pH and prompt pulmonary vascular vasoconstriction. Hyper- or hypo-ventilation can therefore be used to reduce or increase PVR and adjust the Qp/Qs ratio in the required direction.
Intubation
Young children’s pulmonary functional residual capacity (FRC) and oxygen reserve are reduced while metabolic requirements are high. The apnoea period imposed by the intubation procedure should be kept as brief as possible and preceded by a period of hyperventilation using a mask. Moreover, it is essential to reduce PaCO2 in the event of pulmonary hypertension. However, high intrathoracic pressure (forced positive-pressure ventilation, Valsalva manoeuvre) forces a bidirectional shunt in the R-to-L direction (PRA > PLA). Children with predominantly L-to-R ASDs may suddenly desaturate or embolise at arterial level when ventilated at an excessive positive pressure. Since hyperventilation renews the alveolar air more quickly than the pulmonary blood flow, the alveolar concentration of anaesthetic gas is increased and therefore its blood concentration also rises. This induction-related acceleration is more pronounced for soluble agents (halothane) than for agents with low solubility (desflurane) – isoflurane and sevoflurane are middle-ranking in this respect [31].
Nasal intubation is preferable for children weighing up to 5 kg or in cases of prolonged intubation for children aged up to 2 years. Given the occasional need to institute hyperventilation and due to low pulmonary compliance, an internal tube diameter that prevents leakage below a pressure of 20 cm H2O is required. A cuffed tube ensures air-tightness and is recommended in all cases, except for neonates [2]. In 25% of cases, cardiac malformations are combined with other malformations. Some subjects also have facial dysmorphism, which makes intubation difficult. Anaesthetists must plan for this in the preoperative examination to avoid being unexpectedly faced with a cyanotic, curarised child who is difficult to ventilate and moreover has an invisible glottis!
High-volume, rapid administration of fentanyl often prompts rigidity and choreoathetotic movements through extrapyramidal stimulation. Mask ventilation without curare is therefore difficult or even impossible. Bradycardia reduces cardiac output. This situation can rapidly become dangerous. The best solution is rapid muscle relaxation with 0.3 mg/kg of vecuronium or 1.2 mg/kg of rocuronium (time required until intubation is possible: 35 seconds), or possibly 1-2 mg/kg of succinylcholine (time to intubation: < 15 seconds) [28].
Ventilation
The reduction in pulmonary compliance and increase in airway resistance in children with L-to-R shunting and increased pulmonary blood flow is proportionate to vascular overload. The correlation with PAP is less evident [29]. The pulmonary vessels may compress bronchi and restrict flow due to their excessive size.
Positive-pressure ventilation negatively impacts the right heart and has a positive effect on the left heart. PVR is minimal if the pulmonary volume matches the functional residual capacity (see Figure 14.19) [9]. Hypoxia and hypercapnia lower local pH and prompt pulmonary vascular vasoconstriction. Hyper- or hypo-ventilation can therefore be used to reduce or increase PVR and adjust the Qp/Qs ratio in the required direction.
- Hyperventilation (PaCO2 30-35 mmHg) with high FiO2 (≥ 0.8) in the event of PAH for lowering PVR;
- Hypoventilation (PaCO2 45-50 mmHg) with low FiO2 (0.21-0.3) for reducing excessive pulmonary blood flow (Qp > Qs).
In any situations where right-side circulation is impaired (e.g. RV defect, Fontan physiology, etc.), the respirator should be finely adjusted to maintain the lowest possible mean intrathoracic pressure (minimum PEEP to prevent alveolar collapse, I:E ratio of 1:3-4), while hyperventilating the child to reduce local pH, generally with a low tidal volume (< 10 mL/kg) and a high rate. If NO is added even at a low dosage (10 ppm), this reduces PVR by up to 30% [12].
In volume-controlled ventilation, it is possible to administer the same tidal volume (TD) even if conditions change (compliance, relaxation, bronchospasm, etc.), although there is a risk of a very high pressure peak. Alarms and pressure limits prevent the pressure from rising excessively high. Although pressure-controlled ventilation offers the benefit of limiting the risk of barotrauma, it administers variable tidal volume depending on the mechanical conditions of the thorax and lungs. It is the preferred option for young children (< 10 kg), particularly since the TD of anaesthesia ventilators is imprecise if the administered volume is low due to the machine's large compressible volume. In these instances, the pressure-controlled technique is more accurate.
Cyanotic episodes
Some situations may trigger cyanotic episodes (blue spells) between induction and CPB, especially in the event of dynamic stenosis of the RV outflow tract. These may be triggered by two mechanisms.
In volume-controlled ventilation, it is possible to administer the same tidal volume (TD) even if conditions change (compliance, relaxation, bronchospasm, etc.), although there is a risk of a very high pressure peak. Alarms and pressure limits prevent the pressure from rising excessively high. Although pressure-controlled ventilation offers the benefit of limiting the risk of barotrauma, it administers variable tidal volume depending on the mechanical conditions of the thorax and lungs. It is the preferred option for young children (< 10 kg), particularly since the TD of anaesthesia ventilators is imprecise if the administered volume is low due to the machine's large compressible volume. In these instances, the pressure-controlled technique is more accurate.
Cyanotic episodes
Some situations may trigger cyanotic episodes (blue spells) between induction and CPB, especially in the event of dynamic stenosis of the RV outflow tract. These may be triggered by two mechanisms.
- Systemic vasodilation increases R-to-L shunting. In most cases, a vasopressor agent is administered (phenylephrine 1 mcg/kg) and the circulating volume is increased (10 mL/kg albumin or colloid).
- Sympathetic stimulation (pain, aortic root dissection on opening the pericardium) may prompt a spasm of the RV outflow tract. This is treated by deepening the anaesthesia and administering a short-acting β-blocker (esmolol, 0.5 mg/kg). The operator can reduce the dissection's sympathetic reactivity by administering lidocaine in the pericardium when opening it.
These measures are combined with hyperventilation (respiratory alkalosis), an increase in circulating volume, and monitoring of ventilation (checking for unilateral intubation and pneumothorax, aspirating secretions, etc.). In cases of pulmonary stenosis/hypoplasia, a reduction in PVR is prevented by hyperventilation, since this procedure increases the gradient through the stenosis.
In bidirectional shunts, pulmonary vasodilation overloads the pulmonary circulation and induces a harmful reduction in systemic blood flow.
Pulmonary hypertension
There are numerous PAH-prone medical conditions in which a hypertensive crisis may easily be triggered by a series of factors that must be avoided at all costs: hypoventilation, hypercapnia, hypoxaemia (PaO2 < 60 mmHg), atelectasis, high intrathoracic pressure, acidosis, hypothermia, sympathetic stimulation and pain. PAH lowers the pulmonary blood flow and intensifies arterial desaturation. It increases RV afterload and results in right ventricular failure. Although anaesthetic agents have a relatively low impact on the pulmonary circulation, they may have an influence in critical situations [10].
In bidirectional shunts, pulmonary vasodilation overloads the pulmonary circulation and induces a harmful reduction in systemic blood flow.
Pulmonary hypertension
There are numerous PAH-prone medical conditions in which a hypertensive crisis may easily be triggered by a series of factors that must be avoided at all costs: hypoventilation, hypercapnia, hypoxaemia (PaO2 < 60 mmHg), atelectasis, high intrathoracic pressure, acidosis, hypothermia, sympathetic stimulation and pain. PAH lowers the pulmonary blood flow and intensifies arterial desaturation. It increases RV afterload and results in right ventricular failure. Although anaesthetic agents have a relatively low impact on the pulmonary circulation, they may have an influence in critical situations [10].
- Halogenated agents: isoflurane and sevoflurane are associated with pulmonary vasodilation. They reduce SVR (especially isoflurane) and prompt myocardial depression in neonates. Desflurane potentiates alpha-sympathetic stimulation and increases PVR.
- Fentanyl: all forms of fentanyl limit vasoconstrictive response to sympathetic stress.
- Etomidate, midazolam: no impact on pulmonary vasoactivity.
- Propofol: reduces preload, which is detrimental to a hypertrophied RV, and lowers SVR, which increases the R-to-L component of a bidirectional shunt.
- Ketamine: increases already high PVR, although this effect is counteracted by any pulmonary vasodilator agents used.
- Thoracic epidural: sympathicolysis impedes the compensatory inotropic response to PAH [25].
Treatment consists of hyperventilation (PaCO2 30-35 mmHg), hyperoxia (FiO2 0.8-1.0), correction of acidosis, inhalation of NO and/or prostaglandin and milrinone (see Pulmonary Hypertension).
Perioperative infusions
Since CPB represents a large fluid input that is often higher than the child’s circulating volume, intravenous administration is minimised.
Perioperative infusions
Since CPB represents a large fluid input that is often higher than the child’s circulating volume, intravenous administration is minimised.
- No infusion prior to CPB;
- Correction of blood volume with albumin or a colloid (gelatine);
- Correction of hypoglycaemia.
If volume needs to be administered to correct hypotension, a colloid or a 5% (10-15 mL/kg) or 20% (5 mL/kg) albumin solution is used and repeated if necessary. Blood is used in the event of anaemia. Although 6% HES and 5% albumin are equivalent in terms of renal function and morbimortality, HES, whose toxicity has not been demonstrated in paediatrics, leads to a less positive fluid balance than albumin [32]. However, albumin is the safest agent for replacing circulating volume.
Fluid maintenance is ensured by an isotonic balanced solution containing sodium and glucose, since young children are prone to hyponatraemia if free water is administered and to hypoglycaemia in the event of fasting [33]. Sufficient blood glucose levels must be maintained throughout operations on young children, even if the physiological stress of surgery prompts hyperglycaemia, which may result in metabolic acidosis, hyperosmolarity (risk of brain haemorrhage), and osmotic diuresis. Traditionally, hyperglycaemia is avoided due due to its potential harmful effects on the central nervous system in the event of low blood flow or circulatory arrest. However, there is no evidence proving that this notion applies to CPB in children. Indeed, this exacerbation does not appear to affect infants aged under 3 months, since the immaturity of their blood-brain barrier protects them from the effects of hyperglycaemia. In contrast, the harmful cerebral effects of any hypoglycaemia add to those of hypoperfusion. Indeed, neonates are at higher risk of hypoglycaemia due to their limited reserves and low gluconeogenesis.
Blood volume management
Fluid overload is often triggered by haemodilution and capillary permeability caused by CPB – tissue is oedematous, gas exchange is impaired, and the child’s weight rises by 7-15%. Mortality rises in tandem with this weight gain, which generally lasts 2 to 3 days into the postoperative phase [4,33]. The main risk factors are young age, CPB duration, clinical status, and cyanosis [27]. Overload > 8% is an independent predictor of prolonged mechanical ventilation and acute nephropathy [13]. Restrictive fluid administration is the best form of prevention against the risk of fluid and sodium overload [26].
Blood volume indices are generally split into two categories: static indices (CVP, PAWP, LVEDA, etc.) and dynamic indices (respiratory variations during IPPV of systolic arterial pressure, pulse pressure, stroke volume, Vmax of aortic flow, IVC diameter, etc.) (see Chapter 6 - Dynamic Indices and Goal-directed Fluid Management). In adults, the predictive value of the former for hypovolaemia is low, while that of the latter is very high. Unfortunately, both are relatively ineffective in children since their thoracic, pulmonary and arterial compliance is very high [11]. Only a > 15% respiratory variation in maximum aortic blood flow velocity appears to be a reliable indicator of hypovolaemia [17].
Management of fluid overload requires a diuretic (furosemide, ethacrynic acid) and haemodialysis in the event of renal failure. Since the severity of fluid excess on initiating dialysis determines the prognosis, the indication for this procedure must be precocious if the diuretics do not provide the anticipated benefit [20].
Transfusion
Young children’s oxygen requirement is higher than that of adults (3 times higher in the case of infants). Whatever the level of saturation, oxygen delivery must be appropriate. It is therefore particularly important to maintain optimal cardiac output and adequate haemoglobin levels if saturation levels are low. Optimal haematocrit is between 50 and 60% for cyanotic children. If this rises above 60% it is advisable to perform haemodilution with exchange of volume for volume as shown in the formula below:
Fluid maintenance is ensured by an isotonic balanced solution containing sodium and glucose, since young children are prone to hyponatraemia if free water is administered and to hypoglycaemia in the event of fasting [33]. Sufficient blood glucose levels must be maintained throughout operations on young children, even if the physiological stress of surgery prompts hyperglycaemia, which may result in metabolic acidosis, hyperosmolarity (risk of brain haemorrhage), and osmotic diuresis. Traditionally, hyperglycaemia is avoided due due to its potential harmful effects on the central nervous system in the event of low blood flow or circulatory arrest. However, there is no evidence proving that this notion applies to CPB in children. Indeed, this exacerbation does not appear to affect infants aged under 3 months, since the immaturity of their blood-brain barrier protects them from the effects of hyperglycaemia. In contrast, the harmful cerebral effects of any hypoglycaemia add to those of hypoperfusion. Indeed, neonates are at higher risk of hypoglycaemia due to their limited reserves and low gluconeogenesis.
Blood volume management
Fluid overload is often triggered by haemodilution and capillary permeability caused by CPB – tissue is oedematous, gas exchange is impaired, and the child’s weight rises by 7-15%. Mortality rises in tandem with this weight gain, which generally lasts 2 to 3 days into the postoperative phase [4,33]. The main risk factors are young age, CPB duration, clinical status, and cyanosis [27]. Overload > 8% is an independent predictor of prolonged mechanical ventilation and acute nephropathy [13]. Restrictive fluid administration is the best form of prevention against the risk of fluid and sodium overload [26].
Blood volume indices are generally split into two categories: static indices (CVP, PAWP, LVEDA, etc.) and dynamic indices (respiratory variations during IPPV of systolic arterial pressure, pulse pressure, stroke volume, Vmax of aortic flow, IVC diameter, etc.) (see Chapter 6 - Dynamic Indices and Goal-directed Fluid Management). In adults, the predictive value of the former for hypovolaemia is low, while that of the latter is very high. Unfortunately, both are relatively ineffective in children since their thoracic, pulmonary and arterial compliance is very high [11]. Only a > 15% respiratory variation in maximum aortic blood flow velocity appears to be a reliable indicator of hypovolaemia [17].
Management of fluid overload requires a diuretic (furosemide, ethacrynic acid) and haemodialysis in the event of renal failure. Since the severity of fluid excess on initiating dialysis determines the prognosis, the indication for this procedure must be precocious if the diuretics do not provide the anticipated benefit [20].
Transfusion
Young children’s oxygen requirement is higher than that of adults (3 times higher in the case of infants). Whatever the level of saturation, oxygen delivery must be appropriate. It is therefore particularly important to maintain optimal cardiac output and adequate haemoglobin levels if saturation levels are low. Optimal haematocrit is between 50 and 60% for cyanotic children. If this rises above 60% it is advisable to perform haemodilution with exchange of volume for volume as shown in the formula below:
- Exchanged volume = [ (Htcurrent - Htrequired) / Htcurrent ] • weight • 85 (mL/kg)
As in adults, thresholds for transfusion are restrictive. A value of 80 g/L is a good threshold for non-cyanotic children [23]. In the event of arterial desaturation or severe pulmonary hypertension, it is raised to 100 g/L. Blood administration should ideally be adjusted to SaO2 and SvO2, rather than to a specific level of Hb. Transfusions administered to neonates require blood that is compatible with the mother's blood since maternal antibodies passed through the placenta are detectable up to the 3rd month following birth. If the mother’s blood group is unknown, the infant must be given O-negative, Kell-negative blood [23].
Recovery and extubation
Following a long-standing debate on the subject, the current trend is for shorter periods of postoperative mechanical ventilation and earlier extubation, even for neonates or children with complex pathologies [7,19]. For subjects with the same pathology, there is no significant difference in terms of morbimortality if they are extubated on the operating table or in intensive care a few hours later [24]. Rapid extubation is even possible in difficult situations [1]. However, two different situations may be defined based on constraints related to the type of heart disease and the procedure.
Recovery and extubation
Following a long-standing debate on the subject, the current trend is for shorter periods of postoperative mechanical ventilation and earlier extubation, even for neonates or children with complex pathologies [7,19]. For subjects with the same pathology, there is no significant difference in terms of morbimortality if they are extubated on the operating table or in intensive care a few hours later [24]. Rapid extubation is even possible in difficult situations [1]. However, two different situations may be defined based on constraints related to the type of heart disease and the procedure.
- Situations better suited to rapid extubation (on the table):
- Haemodynamically stable child with no active haemorrhage or arrhythmia;
- Anaesthetic agents and dosages suitable for rapid resumption of spontaneous breathing (fentanyl ≤ 20 mcg/kg, sevoflurane, remifentanil-propofol infusion);
- Analgesia facilitated by local anaesthetic blocks and infiltrations;
- Operation without CPB (coarctation, cavopulmonary anastomosis) or with a short period of CPB without deep hypothermia (T° > 30°C), appropriate rewarming;
- Haemodynamics facilitated by spontaneous ventilation (Glenn, Fontan).
- Situations in which rapid extubation is generally contraindicated:
- Neonates, premature infants, growth retardation, Down's syndrome;
- Hypoxaemia, atelectasis, preoperative respiratory failure;
- Prolonged CPB, long period of aortic clamping, deep hypothermia;
- Haemodynamic instability, LV congestive failure, active haemorrhage;
- Pulmonary hypertension.
Rapid extubation (< 2 hours postoperatively) prevents complications related to mechanical ventilation (barotrauma, respiratory infections, tracheal tube obstruction). It allows early mobilisation, reduces respiratory complications, and shortens the period spent in intensive care [1]. It is an indicator that perioperative problems are under control and offers the benefit of facilitating transport to the postoperative monitoring unit. However, since the operating theatre is immobilised while the patient recovers, this represents an organisational and financial loss compared to extubation within an hour of the patient's arrival in intensive care, since an hour in an operating theatre is more expensive than an hour in intensive care. The next issue is to ensure the child's comfort and analgesia without risking hypoventilation or respiratory failure: morphine (bolus 0.1 mg/kg IV at the end of the procedure), dexmedetomidine (0.2-0.7 mcg/kg/h), intravenous paracetamol [15].
Neuraxial anaesthesia
When combined with general anaesthesia, neuraxial blockade offers three significant benefits (see Chapter 4 - Regional Anaesthesia).
Neuraxial anaesthesia
When combined with general anaesthesia, neuraxial blockade offers three significant benefits (see Chapter 4 - Regional Anaesthesia).
- Superior stress response inhibition than intravenous opiates;
- Facilitation of extubation and spontaneous ventilation in the postoperative phase;
- Improved comfort and analgesia.
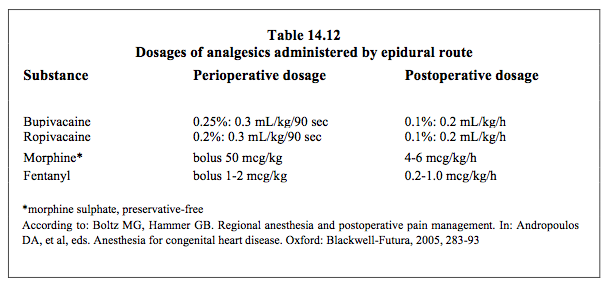
Arterial hypotension, which is a major drawback of neuraxial blockade in adolescents and adults, does not occur in young children due to their immature sympathetic systems – their heart rate does not change significantly [8]. Respiratory depression occurs in children at morphine doses > 0.05 mg/kg administered epidurally and > 0.02 mg/kg administered intrathecally. The major risk is evidently that of a compressive epidural haematoma, especially in children who are anticoagulated for CPB. In adults, calculations of this risk give an epidural haematoma incidence of 1:12,700 to 1:4,000 in cardiac surgery [5,14,18], with a probable mean value of 1:3,550 [16]. Although no precise data are available, there is every reason to expect that the risk is identical in children. A number of precautions aimed at limiting risk are possible. These include leaving at least an hour between puncture and heparinisation and removing the catheter when coagulation is back to normal, which are both simple to achieve with children. However, it is not possible to place the epidural catheter the day before surgery or perform an intrathecal puncture before putting the patient to sleep, as is recommended for adults. The puncture is always performed post-induction on anaesthetised children [3]. The epidural catheter is inserted through a caudal puncture (in very young children) or T4-T8 thoracic puncture (in children > 4 years). The recommended opiate and local anaesthetic doses are given in Table 14.12. The primary indications for an epidural are procedures involving a thoracotomy as this is more painful than a sternotomy, and procedures without CPB, after which the aim is to establish early spontaneous ventilation (e.g. Glenn or Fontan cavopulmonary anastomosis).
In young children, caudal anaesthesia with morphine (70 mcg/kg in 5-10 mL NaCl 0.9% preservative-free) post-induction provides excellent postoperative analgesia.
Peripheral blocks
Ultrasound-guided deep and superficial blocks are much safer than the conventional blind technique. These blocks are particularly useful in case of thoracotomy. The dosage of systemic opiates can be reduced, the children can be extubated immediately at the end of operation, and the postoperative analgesia is excellent [21,22].
- Serratus anterior plane block (thoracotomy);
- Erector spinae plane block (ilio-costal, spinal, and longissimus dorsi muscles);
- Pectoral nerve block (median and lateral);
- Paravertebral plane block.
Various local blocks and infiltrations performed by the surgeon at the end of surgery are extremely useful as they are both effective and present a low level of risk post-CPB, unlike an epidural block.
- Intercostal block: surgeon-performed infiltration of bupivacaine 0.5% (2.5 mg/kg) or ropivacaine 0.2% (3 mg/kg, 2 mg/kg < 1 year) in the intercostal spaces;
- Local infiltration of the sternal wound and the subcutaneous tissue with bupivacaine or ropivacaine;
- Single-shot thoracic paravertebral block performed by the anaesthetist post-induction or at the end of surgery (pre-extubation);
- Continuous paravertebral block (48-72 hours) with ropivacaine 0.2% infusion (0.4 mg/kg/h, 0.2 mg/kg/h < 6 months); puncture by 18-gauge Tuohy needle and catheter at the T5/6 or T6/7 paravertebral spaces at least 1 hour prior to CPB heparinisation [30].
| Anaesthetic technique |
|
Low-risk situations or those suitable for rapid extubation (in the first 4 hours)
- Operation without CPB or with a brief period of CPB (< 90 min) without hypothermia (T° > 34°C) - Appropriate anaesthetic agents and dosages to ensure rapid resumption of spontaneous breathing - Fentanyl ≤ 20 mcg/kg, sufentanil < 2.5 mcg/kg sevoflurane/isoflurane/propofol infusion High-risk situations requiring postoperative ventilation - Young children, growth retardation, severe cyanosis, PAH - Prolonged CPB, long period of aortic clamping, deep hypothermia - Haemodynamic instability, LV or RV congestive failure - Fentanyl 50-75 mcg/kg, midazolam Ventilation: hyper- or hypoventilation depending on whether low or high PVR is required. Mechanical ventilation, preferably pressure-controlled Limitation of fluid and electrolytes (2-4 mL/kg/h depending on Ht) Infants are more at risk of hypo- than hyperglycaemia Field blocks and local anaesthetic infiltrations improve postoperative analgesia and enable fast-track extubation |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- ALGHAMDI AA, SINGH SK, HAMILTON BCS, et al. Early extubation after pediatric cardiac surgery: systematic review, meta-analysis, and evidence-based recommendations. J Card Surg 2010; 25:586-95
- ANDROPOULOS DA. Heart and lung transplantation : anesthetic considerations. In : BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1792-806
- BOLTZ MG, HAMMER GB. Regional anesthesia and postoperative pain management. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 283-93
- BONTANT T, MATROT B, ABDOUL H, et al. Assessing fluid balance in critically ill pediatric patients. Eur J Ped 2015; 174:133-7
- CHAKRAVARTHY M, THIMMANGOWDA P, KRISHNAMURTY J, et al. Thoracic epidural anesthesia in cardiac surgical patients: A prospective audit of 2'113 cases. J Cardiothorac Vasc Anesth 2005; 19:44-8
- CHASSOT PG. Anesthésie en chirurgie cardiaque. In: ECOFFEY C, HAMZA J, MEISTELMAN C. Anesthésiologie pédiatrique. Paris: Flammarion, 1997, 183-202
- DINARDO JA. Con : Extubation in the operating room following pediatric cardiac surgery. J Cardiothorac Vasc Anesth 2011 ; 25 :877-9
- FINKEL JC, BOLTZ MG, CONRAM AM. Hemodynamic changes during spinal anesthesiain children undergoing open heart surgery.Anesth Analg 1998; 86:S400
- FISCHER LG, VAN HAKEN H, BÜRKLE H. Management of pulmonary hypertension: Physiological and pharmacological considerations for anesthesiologists. Anesth Analg 2003; 96:1603-16
- FRIESEN RH, WILLIAMS GD. Anesthetic management of children with pulmonary arterial hypertension. Pediatr Anesth 2008; 18:208-16
- GAN H, CANNESSON M, CHANDLER JR, et al. Predicting fluid responsiveness in children: a systematic review. Anesth Analg 2013; 117:1380-92
- GOTHBERG S, EDBERG KE. Inhaled nitric oxide to newborns and children after congenital heart surgery on cardio-pulmonary bypass. A dose-response study. Scand Cardiovasc J 2000 ; 34 :154-8
- HASSINGER AB, WALD EL, GOODMAN DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med 2014; 15:131-8
- HO AM, CHUNG DC, JOYNT GM. Neuraxial blockade and haematoma in cardiac surgery: estimating the risk of a rare adverse event that does not (yet) occured. Chest 2000; 117:551-5
- IODICE FG, THOMAS M, WALKER I, et al. Analgesia in fast-track paediatric cardiac patients. Eur J Cardiothorac Surg 2011 ; 40 :610-3
- LANDONI G, ISELLA F, GRECO T, et al. Benefits and risks of epidural analgesia in cardiac surgery. Br J Anaesth 2015; 115:25-32
- LEE JH, NO HJ, KIM HS, et al. Prediction of fluid responsiveness using non-invasive cardiac output monitor in children undergoing cardiac surgery. Brit J Anaesth 2015; 115:38-44
- MEHTA Y, ARORA D. Benefits and risks of epidural analgesia in cardiac surgery. J Cardiothorac Vasc Anesth 2014; 28:1069-75
- MITTNACHT AJC. Pro : Early extubation following surgery for congenital heart disese. J Cardiothorac Vasc Anesth 2011 ; 25 :874-6
- MODEM V, THOMPSON M, GOLLHOFER D, et al. Timing of continuous renal replacement therapy and mortality in critically ill children. Crit Care Med 2014; 42:943-53
- MONOHAN A, GUAY J, HAJDUK J, et al. Regional analgesia added to general anesthesia compared with general anesthesia plus systemic analgesia for cardiac surgery in children: a systematic review and meta-analysis of randomized clinical trials. Anesth Analg 2019; 128:130-6
- NASR VG, GOTTLIEB EA, ADLER AC, et al. Selcted 2018 highlights in congenital cardiac anesthesia. J Cardiothorac Vasc Anesth 2019; 33:2833-42
- POUARD P, MAURIAT P, LABORDE N, BOURDARIAS B. Cicrulation extracorporelle en chirurgie cardiaque pédiatrique chez le nouveau-né, le nourrisson et l’enfant. In: JANVIER G, LEHOT JJ, eds. Circulation extracorporelle: principes et pratique, 2ème édition. Paris: Arnette (Groupe Liaison SA) 2004, 481-506
- PREISMAN S, LEMBERSKY H, YUSIM Y, et al. A randomized trial of outcomes of anesthetic management directed to very early extubation after cardiac surgery in children. J Cardiothorac Vasc Anesth 2009; 23:348-57
- REX S, MISSANT C, SEGERS P, et al. Thoracic epidural anethsesia impairs the hemodynamic response to acute pulmonary hypertension by deteriorating right ventricular – pulmonary arterial coupling. Crit Care Med 2007; 35:222-9
- RIZZA A, ROMAGNOLI S, RICCI Z. Fluid status assessment and management during perioperative phase in pediatric cardiac surgery patients. J Cardiothorac Vasc Anesth 2016; 30:1085-93
- SEGUIN J, ALBRIGHT B, VERTULLO L, et al. Extent, risk factors and outcome of fluid overload after pediatric heart surgery. Crit Care Med 2014; 42:2591-9
- SLOAN MH, LERMAN J, BISSONNETTE B. Pharmacodynamics of high-dose vecuronium in children during balanced anesthesia. Anesthesiology 1991; 74:656-9
- STAYER SA, HAMMER GB. Airway and ventilatory management. In ANDROPOULOS DB, et al, Eds. Anesthesia for congenital heart disease. Malden (MA): Blackwell Futura (USA), 2005, 266-82
- TAHARA S, INOUE A, SAKAMOTO H, et al. A case series of continuous paravertebral block in minimally invasive cardiac surgery. JA Clin Rep 2017; 3:45
- THORSTEINSSON A, JONMARKER C, LARSSON A, et al. Functional residual capacity in anesthetized children: normal values and values in children with cardiac anomalies. Anesthesiology 1990; 73:876-81
- VAN DER LINDE P, DUMOULIN M, VAN LERBERGHE C, et al. Efficacy and safety of 6% hydroxyethyl starch 130/0.4 (Voluven) for perioperative volume replacement in children undergoing cardiac surgery: a propensity-matched analysis. Crit Care Med 2015; 19:87-97
- WHITING D, YUKI K, DINARDO JA. Cardiopulmonary bypass in the pediatric population. Best Pract Res Clin Anaesthesiol 2015; 29:241-56
