Video: Transgastric short-axis view of the LV in a case of tight aortic stenosis; concentric hypertrophy is massive, the LV cavity is narrowed.
Systolic function
The ejection fraction (EF) is normal or even supranormal (EF > 0.7) because the telesystolic volume is very small. However, systolic output per unit of myocardial mass is not increased and may even be reduced. The duration of ejection is prolonged (tej > 250 msec) because of the constriction at the LV outlet, which reduces the ventricle's nominal output. As the usual criteria for assessing systolic function depend on loading conditions, it is difficult to quantify the true contractility of the ventricle when filling pressures are high due to diastolic dysfunction and afterload is greatly increased by resistance to ejection. Ventricular dimensions are a better functional criterion: a short-axis LV end-diastolic diameter greater than 4 cm/m2 indicates decompensation. In an asymptomatic stage, tissue Doppler shows subtle changes in contraction: reduction of subendocardial longitudinal contraction, exaggeration of systolic torsion, reduction of protodiastolic suction (see haemodynamic tests, functional indices) [3].
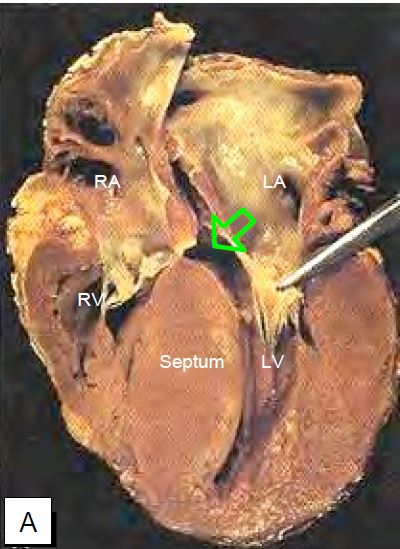
Figure 11.105: Pathological images of concentric left ventricular hypertrophy in narrow aortic stenosis. A: Longitudinal section of the LV; massive hypertrophy of the interventricular septum is seen; the forceps lift the anterior leaflet of the mitral valve and the arrow indicates the narrowing of the outflow tract. B: Cross-section (short axis) of the LV.
The stroke volume is limited by the flow through the stenosis and the duration of systole. It is relatively fixed as there is no contractile reserve. Systemic arterial pressure, which is the product of cardiac output and systemic arterial resistance (SAP ≈ CO · SAR), depends essentially on the SAR, which must remain high.
When compensated, an LV with narrow aortic stenosis presents concentric LVH with a small ventricular cavity and high EF. It generates a high transvalvular gradient:
- ΔPmean > 40 mmHg;
- Pmax > 100 mmHg;
- Aortic flow velocity > 4.0 m/s;
- Velocity time interval ( VTIVao ) > 100 cm.
Stenosis of the aortic valve is responsible for the acceleration of blood flow. If the stenosis is tight, Vmax is at least four times greater than that of the pressure chamber: the ratio between the velocity in the LVOT ( VTILVOT ) and the velocity through the aortic valve ( VTIVao ) is < 0.25, whereas it is > 0.4 in the case of mild stenosis and > 0.8 in the case of a normal valve [1]. Velocity integral (VTI) is most commonly used in preference to Vmax because it is more reliable and less dependent on haemodynamics.
Narrow aortic stenosis: VTILVOT / VTIVao < 0.25
When LV systolic function decreases or stroke volume is reduced (hypovolemia), the transvalvular gradient is lower for the same degree of stenosis. However, the ratio VTILVOT / VTIVaoremains a reliable assessment of stenosis because variations in flow affect both terms of the equation in the same way; the ratio only changes if the functional valve area varies [6]. If systolic aortic flow increases, as is the case with valvular regurgitation, the gradient will be artificially high for the same degree of stenosis because the ejected volume is greater, but the ratio VTILVOT / VTIVao will remain stable.
Diastolic dysfunction
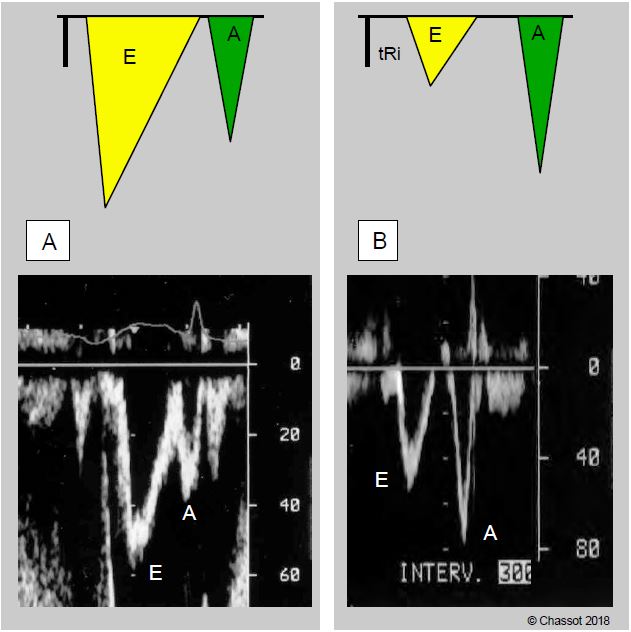
A thick, hypertrophied ventricle is not very compliant. The protodiastolic suction of the active relaxation phase is inadequate. When analysing the mitral flow, the E wave of passive filling has a lower velocity than the A wave of active filling of the atrial contraction (E/A ratio < 1); the isovolumetric relaxation phase (tRI) is prolonged; the deceleration of the E wave (tDE) is shortened (Figure 11.106). The LV, which is very thick, requires a high end-diastolic pressure to reach its filling volume. The PLA increases and the atrium dilates.
Figure 11.106: Changes in mitral flow in concentric ventricular hypertrophy. Mitral flow recorded by Doppler TEE. Left (A), image of normal flow; right (B), image of flow in LVH: A-flow is greater than E-flow, illustrating the dependence of ventricular filling on atrial contraction. The isovolumetric relaxation time (tRi) increases because the mitral valve opens late at the beginning of diastole due to lack of ventricular relaxation; the deceleration time of the E flow is shortened.
As active protodiastolic relaxation is insufficient, the contribution of atrial contraction to ventricular filling becomes increasingly important (30-50% of end-diastolic volume), allowing ventricular end-diastolic pressure to increase without increasing mean atrial or pulmonary venous pressure. This requires a PR interval of at least 0.1 - 0.15 seconds to be effective. The PCWP "a" wave becomes prominent on the Swan-Ganz pulmonary catheter tracing. Loss of sinus rhythm (intraoperative nodal rhythm, transition to atrial fibrillation) collapses cardiac output because the LV suddenly loses 40-50% of its filling mechanism.
Ischaemic risk
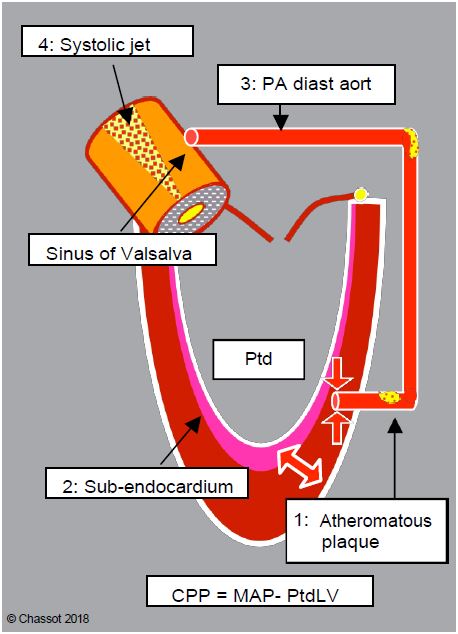
There are a number of factors that explain the increased risk of myocardial ischaemia in aortic stenosis (Figure 11.107) [2,4].
Figure 11.107: Mechanisms of ischaemia in aortic stenosis. 1: Atheromatous plaque. 2: Subendocardial ischaemia related to the thickness of the muscle to be perfused, compression of intramyocardial vessels and high LV end-diastolic pressure (LVdP). 3: A fall in aortic diastolic blood pressure (systemic hypotension) is not accompanied by a fall in LV intracavitary pressure because the aortic stenosis is fixed; coronary perfusion pressure (CPP) falls. 4: During systole, the high-velocity ejection jet (Vmax > 4 m/s) lowers the pressure in the sinuses of Valsalva by the Venturi effect and draws blood from the coronary trunks; systolic perfusion is therefore suppressed (major loss for RV) and diastolic perfusion must begin by filling the epicardial trunks, reducing distal intramyocardial perfusion. 5: Diastole is shortened compared to the prolonged ejection time; moreover, the small stroke volume implies a minimal rate to maintain cardiac output.
- Coexisting coronary artery disease reduces oxygen delivery due to clinically significant stenoses; the association of coronary artery disease and calcified aortic stenosis is common, as both conditions share the same aetiopathogenic basis.
- A fall in arterial pressure is not associated with a fall in intraventricular pressure because the aortic stenosis is fixed; coronary perfusion pressure (CPP) falls without compensation when aortic diastolic BP falls, because CPP = MAP - LVdP.
- The increase in ventricular mass is not associated with a corresponding increase in the coronary network; there is a relative lack of intramyocardial capillary network and coronary reserve is very limited.
- Concentric ventricular hypertrophy is unfavourable for subendocardial coronary perfusion because the wall is thick and therefore takes a long time to cross, and because the muscle compresses the coronary network.
- The slowing of ejection (300-350 msec) caused by the stenosis extends systole and shortens diastole; the ratio of systolic to diastolic time approaches 1:1. On the other hand, the small ejectable stroke volume involves a minimum heart rate high enough to maintain cardiac output.
- The massive increase in pressure work increases myocardial oxygen consumption (mVO2 ) to provide the same external ejection work (see Fig. 11.109). With a ΔPmax of 100 mmHg, the intraventricular systolic pressure must be 200-250 mmHg to maintain systemic perfusion pressure.
- The eddies created at the exit of the valve stenosis of the sinuses of Valsalva form opposite the right and left coronary orifices. As they are areas of depression, these vortices suck the blood into their vortex by the Venturi effect; the flow in the coronary arteries can therefore be reversed during systole.
Clinical adaptations
To ensure adequate ejection in the face of aortic obstruction, the LV makes full use of its preload reserve by moving up and to the right of the Starling curve. In diastolic dysfunction, the compliance curve is very steep, meaning that small variations in preload result in large variations in ejected volume. Clinically, this means that the LV is highly dependent on filling pressures and has no tolerance to hypovolemia. The decrease in venous return associated with induction of anaesthesia or placement of a spinal block results in a significant decrease in cardiac output, which in practice leads to episodes of severe hypotension.
The ability to adapt to peripheral demands by varying cardiac output is limited because stroke volume is relatively fixed and tachycardia is limited by the slowing of the ejection phase and the obligatory duration of diastole to ensure filling. Blood pressure is therefore closely related to peripheral resistance.
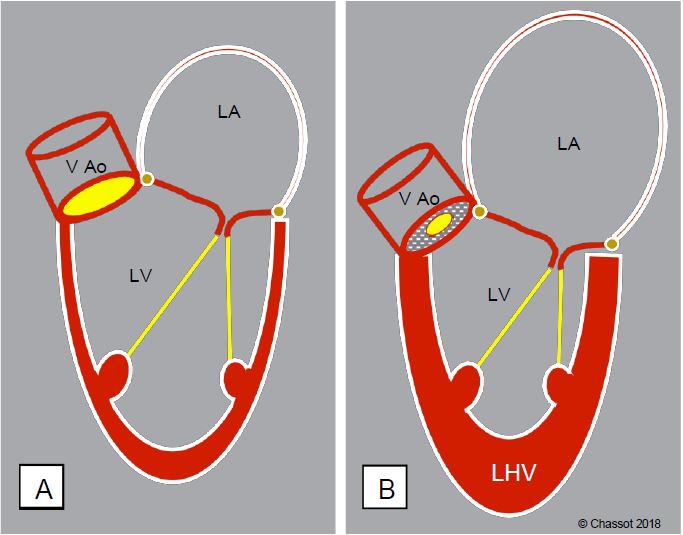
The cardiac silhouette is characterised by a left ventricle with a very thick wall and a small chamber, and a left atrium that is moderately dilated due to the increase in LVdP (Figure 11.108). The pressure-volume loop in pure aortic stenosis is a good illustration of these characteristic changes (Figure 11.109).
Figure 11.108: Schematic representation of the remodelling of the left cavities in narrow aortic stenosis.
A: Normal heart. B: Aortic stenosis; the left atrium is moderately dilated, the LV is concentrically enlarged, its wall is very thick but its cavity is small.
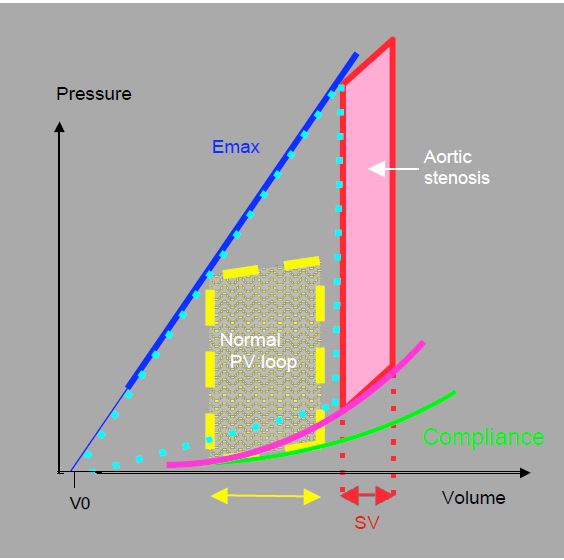
Figure 11.109: Pressure-volume loop in aortic stenosis. The increase in afterload shifts the PV loop upwards and to the right. The slope of the maximum elastance (Emax) is unchanged, but the compliance curve is abnormal. For the same surface area (unchanged external work), the loop is narrower, so the volume ejected (SV: stroke volume) is smaller. While the ejection performance is reduced, the pressure work is greatly increased, as shown by the increase in the area of the triangle between Emax, compliance and the PV loop (light blue dotted line). The dynamic efficiency is greatly reduced even though the developed pressure is higher.
- The increase in afterload moves the PV loop upwards and to the right. The slope of the maximum elasticity (Emax) is unchanged, but the loop becomes narrower to maintain the same surface area (unchanged external work). As a result, the stroke volume (SV) is smaller.
- The systolic ascending slope (isovolumetric contraction) is straight, indicating preserved ejection function; when the myocardium reaches its functional limits (at a telesystolic pressure of 250-300 mm Hg), this slope slopes downwards, the ejection fraction falls and stroke volume is further reduced.
- The diastolic compliance curve is higher and more sloping; for the same diastolic volume, the filling pressure is higher. The PCWP reading is therefore misleading and underestimates the true filling of the LV; a patient may be hypovolemic with a normal PCWP. Echocardiography is a better criterion for assessing blood volume as it gives a direct view of the volume of the ventricles.
- The area of the triangle between the Emax slope, the compliance curve and the P/V loop represents the work of pressure (Tpr); this area is very large, representing a massive increase in mVO2, while the ejected volume is reduced; the efficiency of the myocardium (Téj/Tpr) is very low.
As the valve area becomes smaller, ventricular hypertrophy and wall thickness increase less than the afterload: this situation is known as afterload mismatch [7]. As the ability to compensate is exceeded, systolic function declines. This results in a decrease in functional indices and progressive dilation of the left ventricular chamber.
When aortic insufficiency (AI) is associated with stenosis, a different load is imposed on the ventricle. Aortic insufficiency causes volume overload, which tends to dilate the ventricle. Morphological analysis of the ventricle on TEE provides the anaesthetist with the best means of determining the dominant valve damage.
- If stenosis is predominant, the ventricular chamber is small, the myocardium is very thickened, diastolic dysfunction is significant and the ejection fraction is high (EF > 0.6).
- If insufficiency is predominant, the left ventricle is dilated and spherical, less thickened, diastolic function is less impaired and the ejection fraction is lower (EF: 0.5) due to dilation. Enlargement of the chamber is detrimental to energy output because it forces the LV to work over a large radius of curvature; according to Laplace's law, wall stress increases to develop the same pressure.
Mild to moderate concomitant mitral insufficiency (MI) is common [5]. It is associated with three phenomena.
- The increase in afterload induces intraventricular systolic pressures to which the mitral valve can no longer seal; this component disappears when the aortic stenosis is corrected.
- Ventricular dilatation, which occurs when the LV fails, distends the mitral annulus and spreads the leaflets apart; the leaflets can no longer meet in systole; this restrictive MI is a sign of ventricular decompensation. It gradually resolves when the obstruction to ejection is removed.
- The mitral valve may suffer from its own pathology (fibroelastic degeneration, prolapse), or aortic calcific disease may extend to the anterior leaflet and the mitral annulus, which no longer contract in systole; these elements disrupt the closing mechanisms. This organic component does not change with aortic valve replacement.
| Hemodynamic features of aortic stenosis |
| Concentric LVH, preserved systolic function LV diastolic dysfunction Stroke volume highly dependent on preload Fixed stroke volume (systemic AP dependent on SAR) Cardiac output highly dependent on sinus rhythm and rate High ischaemic risk Criterion for LV dysfunction: end-diastolic diameter > 4 cm/m2 |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BONOW RO, CARABELLO B, CHATTERJEE K, et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. J Am Coll Cardiol 2008; 52:e1-e142
- CARABELLO BA. Clinical practice: aortic stenosis. N Engl J Med 2002; 346:677-82
- GIORGI D, DI BELLO V, TALINI E, et al. Myocardial function in severe aortic stenosis before and after aortic valve replacement: a Doppler tissue imaging study. J Am Soc Echocardiogr 2005; 18:8-14
- GOULD KL, CARABELLO BA. Why angina in aortic stenosis with normal coronary arteriograms? Circulation 2003; 107:3121-4
- MOAZAMI D, DIODATO MD, MOON MR, et al. Does functional mitral regurgitation improve with isolated aortic valve replacement ?. J Card Surg 2004; 19:444-8
- OH JK, SEWARD JB, TAJIK AJ. The echo manual. 3rd edition. Philadelphia, Lippincott Williams & Wilkins, 2006, 189-242
- RUEL M, KULIK A, RUBENS FD, et al. Late incidence and determinants of reoperation in patients with prosthetic heart valves. Eur J Cardiothorac Surg 2004; 25:364-70