Features
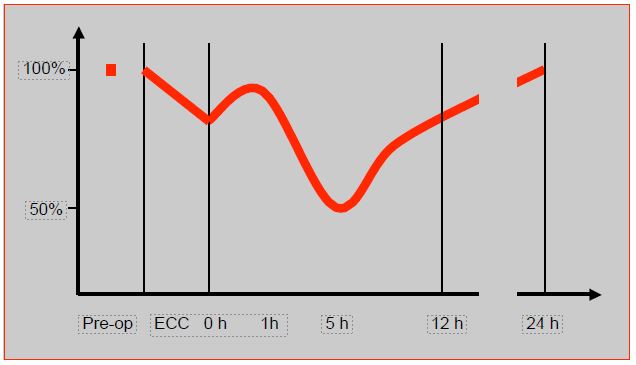
Any surgery with ECC transiently impairs systolic and diastolic myocardial function; this dysfunction will be all the more severe the lower the preoperative function and the higher the risk of the operation performed. Post-ECC dysfunction follows a very specific track: it improves spontaneously during the first hour after weaning, then worsens to reach a nadir between 4th and 6th hours [33]. It is precisely when mediators released (complement fraction C5a, interleukin-6 and -8, TNF, etc) are highest (Figure 7.51).
Figure 7.51: Evolution of cardiac function after coronary artery bypass grafting in ECC [33]. This curve is constructed from several references; for this reason, myocardial performance, assessed in different ways and at different times in different studies, is not expressed in units but as a percentage of the preoperative value.
Recovery usually takes 8 to 24 hours, but sometimes several days, and is slower the worse the preoperative function. This situation contraindicates the use of β -blockers to regulate a tachycardia, as they abruptly reveal the underlying dysfunction and lead to haemodynamic collapse. On the other hand, ECC decreases the activity of β1-myocardial receptors by 30%, and proportionally increases that ofa receptors in the sympathetic response to catecholamines [38]. This activity decreases by a further 25% in the immediate postoperative period [34]. Which explains the resistance to amines of β1 type that can be encountered when weaning off pump, and the effectiveness of adrenaline, which has mixed β and α effects .
Many factors are involved in the genesis of post-ECC heart failure. The risk factors are patient-, ECC- and operation-related (Table 7.10).
LV systolic dysfunction is expressed by arterial hypotension, low cardiac output (index < 2.2 L/min/m2 ), increased arteriovenous O difference2 (> 5.5 ml/L), central venous desaturation (SvO2 < 55%), elevated PWCP (> 18 mmHg), LV end-diastolic pressure > 10 mmHg, and often bradycardia. The ventricle is hypokinetic and dilated on echocardiographic examination (Video). While it is 1-2% when the EF is > 0.4, operative mortality of CABG is 9-12% in case of left dysfunction after ECC (EF < 0.35) [42]. Isolated right-sided failure is associated with a mortality up to 8.2% (EF 0.2-0.3) to 16% (EF < 0.2) [4]. When associated with left-sided failure, right ventricular failure results in mortality more than 40% postoperatively [24]. RV function is a better prognostic criterion than PAP value, because a depleted ventricle can no longer generate a high pressure.
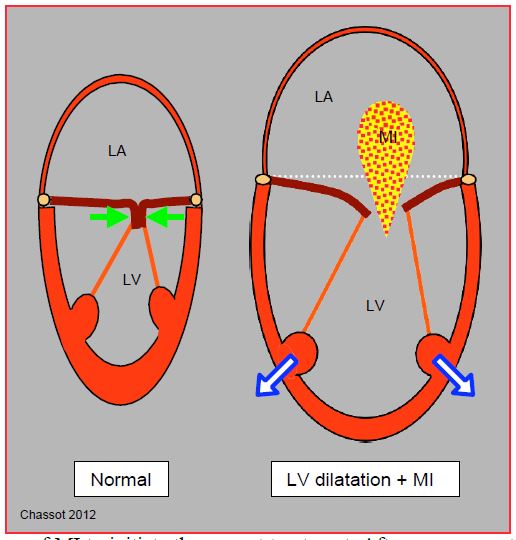
A worsening of mitral insufficiency (MI) is a pathognomonic sign, and a good marker of the degree of ventricular dilation and an excellent sign of acute LV dysfunction (Figure 7.52) (Video).
Video : LV failure 4 chambers view 0°; dilated rounded hypokinetic ventricle (EF 15%); basal lateral segment only has a significant activity; poor mitral valve moves. Normal RV.
Video : LV failure long axis 130° view. VAD mandatory.
Video : biventricular failure; dilated rounded LV; restrictive mitral insufficiency. Dilated RV free wall doesn't contract sufficiently. RV apex is made of RV.
Video : Major mitral insufficiency coming along acute anterior ischemia (mid esophagus 2 chambers 90° view) following CABG
Figure 7.52: Normally, the mitral leaflets face each other with their free edges over a certain height (8-10 mm): their tightness is maintained through intraventricular pressure which presses them together (green arrows). LV insufficiency leads to 3 phenomena: sphericalization of the LV, its dilation, and dilation of the mitral annulus. Splitting of the pillars no longer gives the cords sufficient travel to allow mitral leaflets to meet; the latter are then held below the plane of the mitral annulus during systole, and the valve leaks; mitral insufficiency is said to be restrictive. The extent and variation of mitral insufficiency (MI) is a good criterion for the degree of left ventricular insufficiency.
However, MI after ECC, or the worsening of a previous MI, may indicate several conditions with different treatments and an important differential diagnosis (see Figure 11.53).
- LV dilation: enlarged and dysfunctional ventricle, central MI grade ≥ II (Video). Treatment: catecholamines, inodilators, haemodynamic support on bypass, intra-aortic balloon pump (IABP).
- Excess afterload: central MI. Treatment: vasodilator.
- Segmental ischaemia causing mitral abutment dysfunction (Video). Treatment: nitroglycerin and noradrenaline, haemodynamic support on bypass, IABP, bypass surgery; anticalcium (Dilzem® ) if coronary spasm on internal mammary.
- Mitral prolapse: eccentric MI with tilting of a leaflet in the LA (Video). Treatment: surgical repair, reduction of afterload.
- Dynamic obstruction of the LVCC: aspiration of the anterior leaflet of the mitral valve into the LV outflow tract and mesosystolic reopening of the mitral valve. Origin: hypovolaemia, decreased afterload and sympathetic over-stimulation in a patient with concentric LV hypertrophy (Video). Treatment: interrupt catecholamines, hypervolaemia, vasoconstriction, beta-blockade.
- After mitral plasty: judge the usefulness of a new reconstruction; only a grade I MI is acceptable (Video).
Video : severe restrictive MI following LV failure and dilation
Video : Anterior prolapse following ischemia-rupture of the anterior papillary muscle
Video : severe eccentric MI following posterior leaflet prolapse; MI flows against inter auricular segment
Video : Dynamic sub obstruction of the left outflow tract by anterior mitral leaflet move in mesosystole; leaflet bends and comes in contact with the interventricular septum while in systole (long axis 140° view). Which leads to a partial MV opening leaking in the LA in systole.
Video : MV view after plasty-resection of the posterior leaflet; anterior leaflet only completes the closure; it comes up against the posterior prosthetic ring; no MI.
It is essential to explain the mechanism of MI to initiate the correct treatment. After coronary artery bypass grafting, MI and ventricular dysfunction should raise the suspicion of acute ischaemia, which will show up on TEE as segmental akinesias (Video) and on ECG as ST segment elevations (see Figure 7.49).
Diastolic dysfunction comes along these events in half of the cases; it is sometimes present alone if systolic function is satisfactory. Its incidence varies between 10% and 54% of cases, which represents a prevalence 5-7 times higher than preoperatively [36]. It is reflected by increased filling pressures compared to the actual level of blood volume and a very accentuated respiratory variation seen on the arterial pressure curve [6]. Its intensity has a prognostic value for the immediate functional evolution [3]. This decrease in compliance is due to several phenomena.
- Myocardial oedema (fluid accumulation in the bypass, lack of lymphatic drainage in depulsed flow);
- Cardioplegia, hypothermia;
- Handling of the heart;
- Myocardial ischaemia, reperfusion syndrome, myocardial sideration;
- Systemic inflammatory syndrome;
- Worsening of previous diastolic dysfunction: LVH, ischaemia, cardiomyopathy;
- External compression (pericardium, lungs, pneumothorax).
In addition to these phenomena, consider the haemodynamic consequences of weaning off pump and closing the chest wall.
- Positive pressure ventilation (pulmonary compliance is lowered at the end of the bypass procedure due to alveolar-capillary oedema);
- Volumetric variations (haemorrhages, hypovolaemia);
- Closure of the pericardium and sternum (external compression causing a "tamponade effect");
- Warming (increased O2 consumption, shivering).
In valve corrections, the momentary haemodynamic worsening depends on the type of pathology. Lesions that have led to ventricular dilation (aortic or mitral insufficiency) induce severe dysfunction. After correction of mitral insufficiency, the LV is in a difficult situation because of the sudden increase in afterload due to the removal of the "pressure valve" represented by the valve insufficiency; it also suffers from a drop in preload (backward movement on the Starling curve) secondary to the disappearance of the diastolic return of the regurgitant volume. In the case of mitral stenosis, the problem is related to the small left ventricular volume, whose distensibility is diminished. In contrast, the immediate decrease in afterload after correction of aortic stenosis ensures rapid functional recovery, as long as the ventricular hypertrophy has not impeded myocardial preservation. AVR for tight stenosis and ASD cure are practically the only circumstances where the LV is in a better situation immediately after ECC than before.
Treatment of left ventricular failure
The treatment of post-ECC heart failure focuses on several interdependent points of impact: preload adequacy, afterload reduction (systemic and pulmonary), inotropic stimulation, regulation of cardiac rhythm, and circulatory support [13,22].
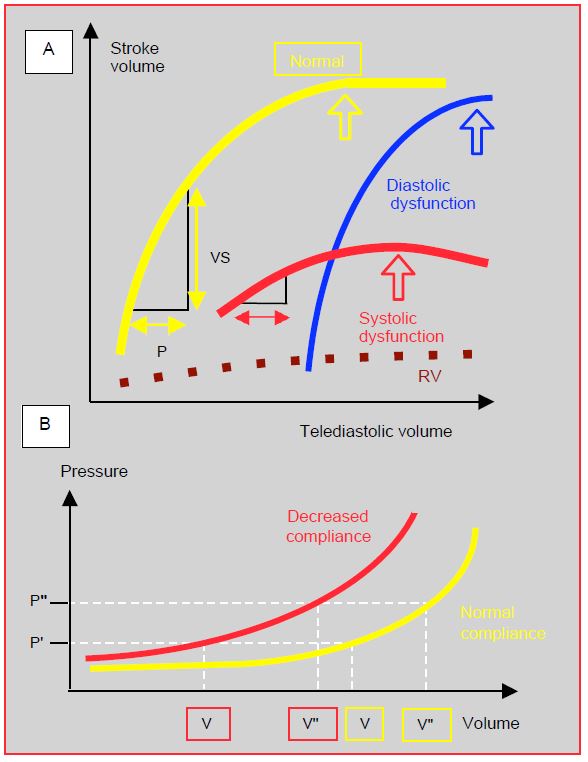
- Preload: the common diastolic failure after bypass surgery changes the compliance curve of the heart chambers; filling pressures (CVP, PWCP) are higher for the same intracavity volume. As the Starling curve of diastolic failure is very steep, stroke volume is dependent on preload, and tolerance to hypovolaemia is very low. In contrast, the curve for systolic failure is flat, reflecting the lack of effect of increased preload on stroke volume. In any case, the increase in preload is limited by the risk of ventricular dilation, a particularly dangerous situation because it increases mVO2 , threatens subendocardial flow and collapses cardiac output (Figure 7.53).
Figure 7.53: Physiological relationships of preload. A: Frank-Starling curves of the LV illustrating the relationship between preload and systolic performance. Normal curve (yellow), curve in systolic dysfunction (red), and in diastolic dysfunction (blue). The RV Starling curve (dotted line) is very flat, which means that normal RV flow is only slightly dependent on preload. B: Normal LV compliance curve (yellow) and in diastolic dysfunction (red). In this second case, the pressure P corresponds to a smaller ventricular volume (V') than the norm (V); the subject may be hypovolaemic with a normal POD (CVP) or POG (PCWP). Normovolaemia in a subject with diastolic dysfunction (V'' red) is a filling pressure (P') that corresponds to hypervolaemia (V'' yellow) in a normal subject.
- Low afterload: Vasoplegia occurs in 8-20% of cases after ECC. Causes are many: preoperative ACEI, diabetes, long ECC, ischaemia-reperfusion injury, SIRS, protamine, etc.; ECC involves elevated NO secretion and lowered vasopressin production [19,35]. Although beneficial to the LV, this decrease in afterload should be limited as it compromises perfusion to the coronaries and all organs. Usual treatment:
- Phenylephrine (Neosynephrine® ): bolus 100 mcg; the maximum recommended dose outside ECC is 1 mg; explained by the complete lack of beta inotropic effect of the substance, which only increases SVR and LV afterload, which could lead to left-sided failure.
- Nor-adrenaline (Arterenol® ): infusion 0.02-0.5 mcg/kg/min; stimulation of a myocardial receptors (positive inotropes) and low β1 effect provide inotropic support to help the LV as its afterload increases.
- Vasopressin (Pitressin® ): In refractory distributive shock (MAP < 55 mmHg, ABP < 600 dynes-s-cm-5 ), vasopressin (1-4 U/h) improves the situation [20]. At these doses, vasopressin causes less coronary, renal and splanchnic vasoconstriction than noradrenaline for the same result on systemic pressure; it does not increase ABP [29]. On the other hand, it retains its vasoconstrictive properties despite hypoxia and acidosis [30].
- Methylene blue (1-2 mg/kg iv over 20 minutes): can restore vascular tone in less than 2 hours in situations where other vasopressors have been ineffective [18,25], but risky at high doses (>7 mg/kg (haemolysis, methaemoglobinaemia, pulmonary hypertension, neurotoxicity, coronary and renal vascoconstriction) [43]. Its benefit is greatest if given early in the onset of hypotension [25]. Skin, conjunctiva and urine appear bluish; pulse oximetry is no longer reliable and gives lower than true SaO2
- High afterload: the risk is dilation of the LV, disruption of the aortic sutures and increased blood loss. The dysfunctional LV is particularly sensitive to increased impedance. The vasodilators used in the first line are predominantly arteriolar [21].
- Deepening anaesthesia (isoflurane 2-4%, propofol) and analgesia.
- Phentolamine bolus (Regitine® 1-2 mg iv).
- Nicardipine (Loxen® , Cardene®5 mg/min iv).
- Clevidipine (Cleviprex 1-2 mg/h, double every 3-5 minutes until desired effect; max dose 32 mg/h).
- Nitroprusside infusion (Nipruss®1-5 mcg/kg/min).
- Nitroglycerin (bolus 50-100 mcg, infusion 5-5.0 mcg/kg/min) if hypertension is associated with preload elevation or PAH.
- An inodilator such as a phosphodiesterase-3 inhibitor (milrinone) or levosimandan is indicated when hypertension is associated with ventricular dilation.
- Rhythm control: Arrhythmias should be treated electrically as soon as possible. The ideal rhythm weaning off ECC is a sinus rythm and a 75-80 beats/minute frequency .
- In case of AV dissociation: bicameral pacemaker, isoprenaline (10 mcg bolus). Synchronisation of atrial contraction is even more important if alterations in ventricular compliance.
- AF or supra-junctional tachyarrhythmia: intraoperative cardioversion (2-10 J by internal pads); cardioversion is worth trying, even in a patient with chronic AF, as sinus rhythm, even temporarily, improves haemodynamics during hours when it is most compromised. The first-line drug is amiodarone (Cordarone® ), infused at 10-15 mg/kg/24h; start with 150 mg/20 minutes iv, repeat as needed. Esmolol (Brevibloc® ) is the only β-blocker that can be considered postoperatively because of its short half-life (9 minutes); however, it should be used cautiously and only when tachycardia is excessive or dangerous in case of normal ventricular function.
- Torsade de pointes, VT, coronary spasm: internal (5-50 J) or external (100-360 J) defibrillation, xylocaine (1 mg/kg), magnesium (loading: 1-2 gm iv).
- Inotropic pacing: If the cardiac index is less than 2.2 l/min/m2 and the PAPO is greater than 18 mmHg, or the ejection fraction is less than 0.5, inotropic support is required. The choice of cardiostimulatory agents is described below and in Tables 7.11, 12 and 7.13.

In the operating theatre, drug therapies can easily be reinforced by a ventricular assist system, which maintains circulation by supporting the ventricle(s) thus providing an opportunity for recovery (see Chapter 12 Ventricular Assist).
- Intra-aortic balloon pump (IABP): decreases LV afterload and increases diastolic pressure, thus coronary perfusion. First choice mean to support a failing LV due to dilation (presence of MI grade > II/IV) or ischaemia, effective only if the rhythm is regular and below 120 beats/min, and if systemic resistance is maintained. It is contraindicated in cases of aortic insufficiency, dissection, or severe atheromatosis of the descending thoracic aorta.
- ECMO (Extra-Corporeal Membrane Oxygenation): a centrifugal pump (see Pumps) coupled to an oxygenator and connected to the femoral vessels provides assistance to ventricular function and gas exchange; it is indicated for 3-5 days in case of cardio-respiratory failure.
- External ventricular assist with a short-term system, such as the Abiomed BVS 5000i™ (pulsatile flow), Impella™ (continuous flow) or TandemHeart™ (continuous flow); the latter two are introduced percutaneously under fluroscopic and/or echocardiographic guidance (see Percutaneous Assist).
- Regardless of the type, ventricular assist is not a substitute for inotropic agents or preload maintenance. Indeed, right-sided failure is common with isolated left-sided support, hence the need for NO, milrinone, adrenaline, levosimendan; on the other hand, the RV provides preload for the support system, which is directly dependent on ventricular filling [22].
Inotropic stimulation
Chronic ventricular failure and prolonged stress (long ECC, continuous haemodynamic instability in intensive care) lead to a remodelling of myocardial receptors: decrease in β1 receptors, increase in β2 and α1 receptors (positive inotropes) [11,34]. These data have major impacts on catecholaminergic therapy.
- The response to amines β1 is diminished; dopamine and dobutamine rapidly reach their peak activity. Adrenaline, which stimulates β1,β2 and α1 receptors, is the only catecholamine effective in the treatment of acute decompensation of chronic heart failure or in critical situations after bypass surgery. Norepinephrine (major α1 and secondary β1 effect) has more positive inotropic effect than on a normal heart because of the excess of α1 receptors.
- Chronic nor-adrenaline depletion in the myocardium decreases the effectiveness of indirect amines such as dopamine or ephedrine.
- Phosphodiesterase-3 inhibitors (amrinone, milrinone) are effective in ventricular failure or β-blockade because they act through a receptor-independent of β pathway ; same pattern for noradrenaline.
- The combination of adrenaline and milrinone is the most effective inotropic stimulant on a failing ventricle, left or right.
- The choice of inotropic agent has poor effect on 30-day morbidity and mortality, except for levosimendan in certain specific situations [2,26].
The effectiveness of catecholamines, irrespective of their dosage, therefore depends on several elements.
- The density and sensitivity of membrane receptors;
- The distribution of receptor types;
- Synaptic reuptake activity;
- The availability of sarcoplasmic Ca2+ ;
- Acid-base balance (acidosis inhibits their action);
- The local concentration of the substance, which varies according to the haemodynamics and the site of administration (peripheral versus central route);
- The duration of heart failure or cardiogenic shock.
Inotropic agents are widely used, often routinely and "prophylactically". In reality, they are only indicated according to the probability of dysfunction on exit from ECC. This probability varies between 35% (bypass surgery < 60 minutes, EF > 0.65) and 100% (bypass surgery > 150 minutes, EF < 0.35). If, after weaning, the cardiac index is less than 2.5 l/min/m2 and PAPO greater than 18 mm Hg, or the ejection fraction less than 0.5, inotropic support is required.
Catecholamines
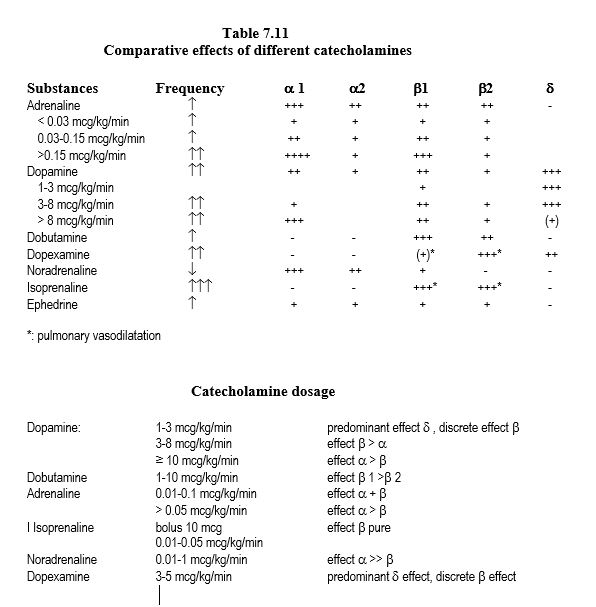
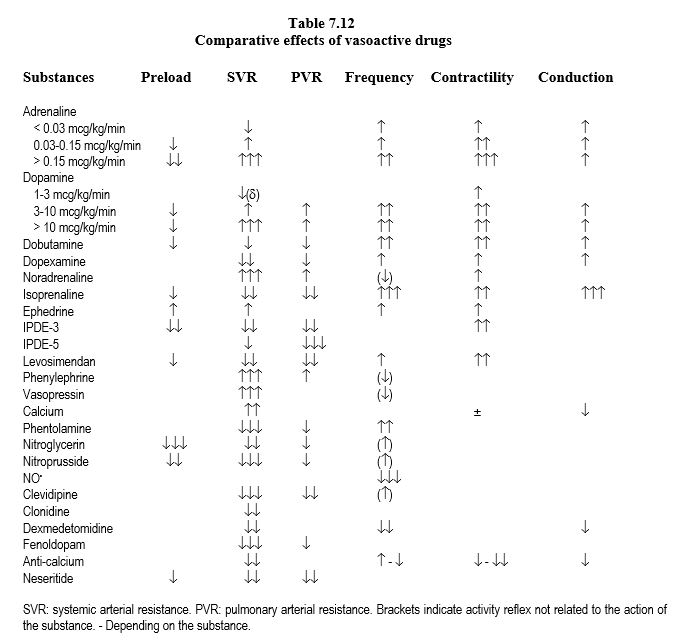
Not all catecholamines are equivalent, as they differ in the selectivity of actions at the receptor site (Tables 7.8, 7.11 and 7.12).
- Dopamine: ideal agent for simple weaning off procedures because of β1 effect, the slight a effect (maintaining SVR) and the d effect (increasing splanchnic and renal flow) if the dosage remains < 5 mcg/kg/min. Above 5 mcg/kg/min, the α vasoconstriction effect increases more than the β one and dopamine becomes essentially α vasopressor. This variability makes it unsuitable for the management of severe ventricular failure. Dopamine is cheap, but relatively tachycardic at low doses.
- Dobutamine: exclusively β stimulating , tends to lower SVR; effects do not change with dosage, but usually require nor-adrenaline infusion above 5 mcg/kg/min. Dobutamine is more expensive but less tachycardic than dopamine.
- Adrenaline: balanced α and β effect, very cheap; above 0.5 mcg/kg/min, the α effect dominates over the β one it induces hyperglycaemia and acidosis.
- Noradrenaline: mainly a systemic arteriolar vasoconstrictor effect; little pulmonary vasoconstrictor effect because α receptors are scarce in the pulmonary bed. Noradrenaline has a positive inotropic effect in chronic ventricular failure because of the preponderance of α receptors in a dysfunctional myocardium.
- Isoprenaline: the most potent stimulant β 1 and β 2; tachycardia and arterial vasodilator, isoprenaline is indicated primarily in AV block and bronchospasm.
Substances that have several actions, such as dopamine, do not allow the effects to be differentiated from one another, especially when their proportion varies according to dosage. Catecholamines with a "pure" effect (dobutamine, noradrenaline) are easier to adjust according to haemodynamic parameter to be influenced. The β2 effect (dobutamine, adrenaline) is responsible for vasodilation, hyperglycaemia and metabolic acidosis. Regardless of the substance used, the increase in contractility always results in an increase in mVO2 . Only assistive ventricular techniques increase output without increasing cardiac work.
Other substances
An inhibitor of phosphodiesterase-3 (IPDE-3), which catabolises cAMP, increases the cytoplasmic level of cAMP, thus leading to inotropic stimulation by increasing systolic [Ca2+ ]i . This pathway does not pass through the b receptors . IPDEs are inodilators: they have a positive inotropic effect, and a vasodilatory effect on resistance vessels (systemic and pulmonary arteries) and capacitance vessels (large central veins). They do not induce tachycardia (no chronotropic effect). The essential indications are [17].
- Severe left-sided dysfunction with ventricular dilation that may benefit from decreased afterload;
- Right ventricular failure with pulmonary hypertension;
- Severely β receptor-depleted hearts such as long-term ventricular failure, heart transplants, and cases after prolonged bypass surgery;
- β-blocked patients.
Milrinone (Corotrop® ) is infused (0.4-0.75 mcg/kg/min) preceded by a loading dose (50 mcg/kg). The latter causes significant hypotension; one possibility is to inject the loading dose during rewarming phase of ECC procedure, as hypotension is then easily controled. The combination of adrenaline and milrinone is particularly effective in chronic, refractory ventricular failure or PHT.
Levosimendan (Simdax® ) has anti-phosphodiesterase inotropic activity and calcium sensitisation of troponin C and is an arterial vasodilator because it opens KATP channels in arteriolar smooth muscle cells. Levosimendan does not induce tachycardia or increase mVO2 [28]. Clinical indications [39]:
- LV insufficiency with high SAR and/or PVR;
- Right-sided failure with PAH;
- Coronary revascularisation with refractory ventricular failure;
- Low cardiac output in β -blocked or β receptor-depleted patients ;
- When other agents expected ineffective.
It is administered as a single 24-hour infusion (0.1-0.2 mcg/kg/min), after a loading dose of 12-24 mcg/kg; treatment for an adult requires about 12 mg (cost: about €700). Because of its slow onset of action, the infusion is better started at induction [39]. Levosimendan is the only inotropic agent that decreases mortality (20-30% decrease) [31,32].
Thyroxine (T3) (tri-iodo-thyronine) improves ventricular performance by stimulation of adenylyl cyclase (increase in cAMP) and by pathways other than cAMP; it is useful in patients whose neurohumoral system is depleted, such as intensive care patients, organ donors, or after prolonged ECC [27]. Its action is counteracted by anti-calcium drugs. Doses are 0.03-0.5 mcg/kg/min, or 0.0275 mcg/kg in 4 doses. Substitution of this drug displays conflicting results ranging from improvement in CO to no effect [7].
Glucose-insulin-potassium (GIK) solution appears to be beneficial mainly in ischaemic patients with severe ventricular dysfunction and decreased b myocardial receptors [40]. It could reduce myocardial suffering (decrease in CK-MB and postoperative troponins), but does not seem to have an impact on clinical outcome [37]. Nevertheless, a recent study shows that a solution of 20 IU insulin + 10 mmol K+ in 50 mL glucose 40% infused between induction and ECC improves myocardial protection; biventricular function on weaning is higher than placebo, troponin levels are lower, and the rate of cardiorespiratory complications is lower (RR 0.6) [9]. The recommended intraoperative regimen is: insulin 2-4 U/h, K+ 10-20 mmol/h, glucose 20% 10-15 g/h (50-75 ml/h). The aim is to maintain an intraoperative blood glucose level of 6-8 mmol/L.
Calcium (dose: 2-4 mg/kg) raises extracellular Ca2+ and antagonises the effects of intramyocardial hyperkalaemia after cardioplegia, but only improves cardiac function in hypocalcaemic patients (rapid transfusion of citrate blood) or those on anticalcics. It is antagonistic to beta-stimulants and synergistic with a stimulants (pressure increase, but no inotropic effect). Acute hypercalcaemia during revascularisation can cause intracellular overload, worsening ischaemic injury and diastolic dysfunction, stiffening the myocardium (stone heart), and inducing vasoconstriction of arterial grafts [41]. In case of normocalcaemia, the risks are greater than the expected benefits: sinus bradycardia, slowing of AV conduction, antagonism with cAMP stimulants (β-agonists), arterial spasm, increased digitalis toxicity. The only indication for calcium at the end of the bypass is the reversal of residual hyperkalaemia.
The combination of hypovolaemia and infusion of catecholamines with β effect during concentric left ventricular hypertrophy can induce a "CMO effect": as in obstructive cardiomyopathy, constriction of the outflow tract in systole and tilting of the anterior mitral leaflet into the LVCC (SAM: systolic anterior motion) create an obstacle to ejection. This dynamic subaortic obstruction is common after aortic valve replacement for stenosis or after mitral plasty. The diagnosis is made on echocardiography (Video). The more you increase the amines, the worse the low flow situation becomes! The only treatment is a decrease in b amines , an increase in SVR (α amines ), volume filling and possibly the introduction of a β-blockade therapy [23].
Therapeutics for right ventricular failure
Right-sided failure is secondary to a disease of the LV itself (infarction, cardiomyopathy), to a lack of cardioprotection in bypass surgery or to an increase of afterload, which is the most frequent. The pulmonary hypertension after bypass surgery is caused by.
- Worsening of pre-existing PHT;
- Stasis on LV dysfunction, residual mitral valve disease or mitral prosthesis mismatch;
- Hypoventilation, hypoxia, hypercapnia, respiratory acidosis, atelectasis;
- Inflammatory lung syndrome;
- Administration of protamine;
- Excessive PPI and PEEP;
- Mechanical compression of the pulmonary vessels (tamponade, pneumothorax, retractor);
- Pulmonary embolism.
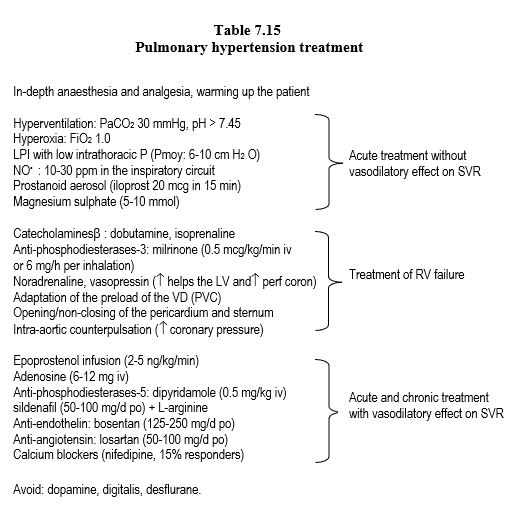
The treatment of right ventricular failure includes several points (Table 7.14) [8,15].
- Optimisation of preload: a dysfunctional RV may require CVP up to 10-12 mmHg to maintain systolic flow. On the other hand, if the failure has already led to ventricular dilation and upstream stasis, preload should be reduced: nitrates, diuretics, counter-Trendelenburg position.
- Decreasing afterload: As the RV is very sensitive to afterload, decreasing impedance effectively increases ejected volume; substances with a major pulmonary vasodilator effect are used for this purpose. Inhaled vasodilators (NO, prostacyclin, milrinone, nitroglycerin), hyperoxia (FiO28-1.0), normobaric hyperventilation and correction of acidosis have no systemic effect, whereas systemic hypotensive agents also lower blood pressure, thereby increasing septal dyskinesia and decreasing right coronary perfusion pressure. Vasodilators are more effective as aerosols than as infusions in decreasing PVRs and improving RV function [10]. On the other hand, inhaled substances are preferentially distributed to well-ventilated areas, whereas systemic vasodilators inhibit hypoxic pulmonary vasoconstriction and result in hypoxaemia. Pulmonary vasodilators (NO, prostacyclins, IPDE-5) are potentially dangerous in pulmonary hypertension related to left stasis (LV failure, mitral disease), as they increase flow and congestion upstream of the RA; the risk is APO. Lowering of PAP is not necessarily a therapeutic success, as it may be due to loss of LV function (Treatment of pulmonary hypertension: Table 7.15).
- Stimulation of contractility: sympathomimetic amines (dobutamine, adrenaline, isoprenaline), phosphodiesterase-3 inhibitors (amrinone, milrinone), calcium sensitiser (levosimandan). Dopamine and digitalis are contraindicated because they increase APR.
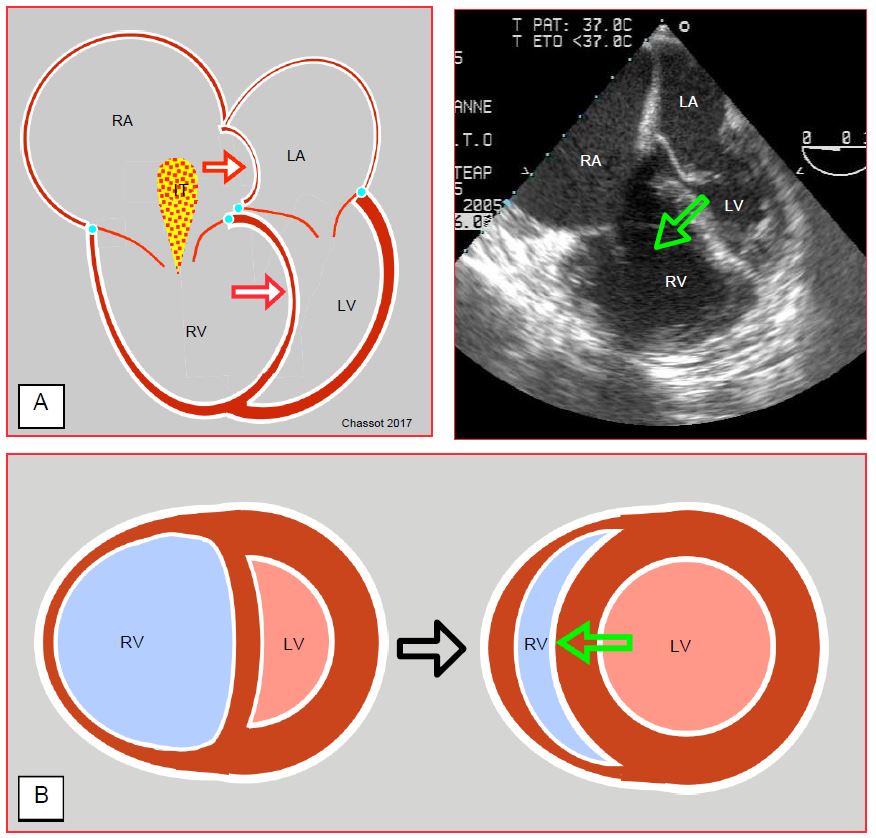
- Maintenance of the pressure gradient between the two ventricles (MAP/PAPm ratio ≥ 0) and of the RV geometry to avoid septal tilt to the left and to support RV contraction. Septal dyskinesia results in 30-40% loss of right ejection efficiency. If the RV becomes globular by bulging to the left, the longitudinal myofibrils, which are the main motor of right ejection, lose their normal alignment and their contraction becomes less efficient. The increase in left afterload (systemic vasoconstrictor) tends to inflate the LV and restore the normal position of the interventricular septum (rounded to the right); thus ejection support to the RV by the LV is restored (Figure 7.54).
Figure 7.54: Right ventricular failure. A: The interventricular septum bulges into the LV and significantly reduces its diastolic volume, as evidenced here by the narrowing of its surface area in diastole (the mitral valve is open). This is the Bernheim effect. The interatrial septum bulges into the RA because of the overpressure in the RA. IT: tricuspid insufficiency. On the right, transoesophageal 4-cavity echocardiographic view (chronic PAH on pulmonary emboli). The aim of systemic vasoconstrictor therapy is to increase LV pressure and volume so that the septum returns to its normal position (green arrow) and contributes to RV ejection as it normally does. B: Short-axis view of the RV-LV couple; in right-sided failure, the septum is pushed back into the LV by RV dilation. The increased LV afterload raises its intraventricular pressure and returns the septum to its physiological position (green arrow).
- Maintenance of right coronary perfusion: nor-adrenaline and/or vasopressin infusion is indicated to maintain a sufficient gradient between aortic and RV systolic pressure; the small population of pulmonary a receptors and the absence of vasopressin receptors in the lungs in the absence of chronic PHT cause systemic pressure to rise but not pulmonary pressure.
- External ventricular support with an artificial ventricle or intra-pulmonary counterpulsation; intra-aortic counterpulsation may be useful in maintaining coronary perfusion pressure.
- Non-closure of the pericardium and sternum, or reopening postoperatively. When the RV Ptd is ≥ 15 mmHg, the ventricular wall abuts the pericardium; opening the pericardium increases RV compliance and relieves the compression on the LV by tilting the interventricular septum. This technique allows the dilation due to right dysfunction to be tolerated without pressure increase.
- Treatment effectiveness is not a decrease in PAP, but a decrease in CVP and an increase in stroke volume (Swan-Ganz catheter).
Impact of inotropic agents on mortality
Despite their clear short-term benefit, routine use of inotropic agents tends to increase mortality as well as the risk of infarction (HR 2.1), stroke (HR 2.4), arrhythmia (HR 3.1) and renal failure (OR 7.9) [26]. After coronary revascularisation, catecholamines tend to worsen ischaemia-reperfusion injury by cytoplasmic Ca2+ overload and increased mVO2 . However, in the specific situation of cardiac surgery, inotropes tend to improve survival (RR 0.73) if their use is reduced to the minimum time and dosage necessary to momentarily maintain cardiac output when weaning off pump [2]. Levosimendan is the only inotropic agent associated with a reduction in long-term mortality (OR 0.35-0.82) in cardiology, intensive care or cardiac surgery [2,16,31].
Magnesium
Mg2+ is the second most abundant intracellular ion. Unfortunately, serum levels are a poor reflection of total magnesium, as only 1% of total Mg is in ionised form in serum. Mg2+ is required for the function of several ATPases and is involved in the maintenance of intracellular K+ and Ca2+ . Hypomagnesemia is very common in heart failure patients, in diabetics, in patients receiving diuretics or digitalis, and at the end of ECC [1]. Therapeutically, magnesium acts as a calcium blocker: it decreases the excess of sarcoplasmic Ca2+ and free radicals associated with ischaemia; it decreases the incidence of ventricular arrhythmias and causes systemic and pulmonary arterial vasodilation. When given before or immediately at the time of reperfusion after a period of ischaemia, it tends to decrease infarct size, improve systolic function, and decrease mortality in patients with preserved left-sided function [44]. The effect is lost if administration is delayed [5].
The side effects, which are uncommon at usual doses, are sedation, muscle paralysis, decreased arterial resistance and inhibition of platelet function [12]. Eliminated by the kidneys, magnesium displays these effects especially in patients with renal failure. Synergism with nitrates and ACE inhibitors can lead to severe hypotension [14]. Because of its safety and low cost, magnesium (MgSO4 or MgCl2 , 2-4 gm iv in adults) is indicated without restriction for ventricular rhythm disturbances or repeated defibrillations; it may be beneficial in arterial vasoconstriction and during ischaemia. Routine administration is usual before and/or after ECC.
| Post-ECC ventricular dysfunction |
| Cardiac intervention in ECC temporarily impairs ventricular function. Systolic and diastolic function decline progressively to reach their nadir at about 5-6 hours after ECC; recovery takes 8 to 24 hours. This dysfunction is all the more severe when a ventricular damage pre-exists associated with a long and complex operation.
Therapeutics for left-sided failure in order of increasing importance:
- Optimisation of preload, afterload, rate and rhythm
- Dopamine up to 5m g/kg/'
- Dobutamine (5-20m g/kg/') + noradrenaline (0.05 - 0.5m g/kg/')
- Adrenaline (0.01-0.3m g/kg/') + milrinone (0.5m g/kg/')
- IABP
- Levosimendan (0.1-0.2m g/kg/min for 24 hours)
- ECMO, ventricular assistance
Therapeutics for right-sided failure:
- Optimisation of VD preload
- Dobutamine (5-20m g/kg/')
- Milrinone (0.5m g/kg/') + adrenaline (0.01-0.3m g/kg/')
- Pulmonary vasodilator: NO, iloprost (inhalation), nitroglycerin (0.1-5.0m g/kg/'),
milrinone (aerosol 6 mg/h)
- Maintenance of ventricular interaction and coronary perfusion: noradrenaline (0.05 - 0.5m g/kg/'), CPIA
- Levosimendan (0.1-0.2m g/kg/min)
- Non-closure of the pericardium and sternum
- Ventricular assistance
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- AGLIO LS, STANFORD GG, MADDI R, et al. Hypomagnesemia is common following cardiac surgery. J Cardiothorac Vasc Anesth 1991; 5:201-8
- BELLETTI A, CASTRO ML, SILVETTI S, et al. The effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Brit J Anaesth 2015; 115:656-75
- BERNARD F, DENAULT A, BABIN D, et al. Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg 2001; 92:291-8
- BOOTSMA IT, DE LANGE F, KOOPMANS M, et al. Right ventricular function after cardiac surgery is a strong independent predictor for long-term mortality. J Cardiothorac Vasc Anesth 2017; 31:1656-62
- BOYD WC, THOMAS SJ. Pro: Magnesium should be administered to all coronary artery bypass graft surgery patients undergoing cardiopulmonary bypass. J Cardiothorac vasc Anesth 2000; 14:339-43
- BRUTSAERT DL, SYS SU, GILLEBERT TC. Diastolic dysfunction in post-cardiac surgical management. J Cardiothor Vasc Anesth 1993; 7(suppl 1):18-20
- CHOI YS, SHIM JK, SONG JW, et al. Efficacy of perioperative oral triiodothyronine therapy to prevent postoperative low triiodothyroninestate following valvular heart surgery. J Cardiothorac Vasc Anesth 2013; 27:1218-23
- DENAULT A, HADDAD F, JACOBSOHN E, et al. Perioperative right ventricular dysfunction. Curr Opin Anaesthesiol 2013; 26:71-81
- ELLENBERGER C, SOLOGASHVILI T, KREIENBÜHL L, et al. Myocardial protection by glucose-insulin-potassium in moderate- to high-risk patients undergoing elective on-pump cardiac surgery: a randomized controlled trial. Anesth Analg 2018; 126:133-41
- ELMI-SARABI M, DESCHAMPS A, DELISLE S, et al. Aerosolized vasodilators for the treatment of pulmonary hypertension in cardiax surgical patients: a systematic review and meta-analysis. Anesth Analg 2017; 125:393-402
- GERHARDT MA, BOOTH JV, CHESNUT LC, et al. Acute myocardial beta-adrenergic receptor dysfunction after cardiopulmonary bypass in patients with cardiac valve disease. Circulation 1998; 98:II275-II281
- GRIES A, BODE C, GROSS S, et al. The effect of intravenously administered magnesium on platelet function in patients after cardiac surgery. Anesth Analg 1999; 88:1213-9
- GRIFFIN MG, HINES RL. Management of perioperative ventricular dysfunction. J Cardiothorac Vasc Anesth 2001; 15:90-106
- GRIGORE AM, MATHEW JP. Con: Magnesium should not be administered to all coronary artery bypass graft surgery patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2000; 14:344-46
- HARJOLA VP, MEBAZAA A, CELUTKIENE J, BETTEX D, et al. Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the working group on pulmonary circulation and right ventricular function of the European Society of Cardiology. Eur J Heart Fail 2016; 18:226-41
- HARRISON RW, HASSELBLAD V, MEHTA RH, et al. Effect of levosimendan on survival and adverse events after cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth 2013; 27:1224-32
- KIKURA M, SATO S. The efficacy of preemptive milrinone or amrinone therapy in patients undergoing coronary artery bypass grafting. Anesth Analg 2002; 94:22-30
- LAVIGNE D, Vasopressin and methylene blue: alternate therapies in vasodilatory shock. Semin Cardiothorac Vasc Anesth 2010; 14:186-9
- LEVIN MA, LIN HM, CASTILLO JG, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation 2009; 120:1664-71
- LICKER M, SCHWEIZER A. Vasopressin and postcardiopulmonary bypass refractory hypotension. Anesth Analg 1999; 88:695
- LIU H, FOX CJ, ZHANG S, KAYE AD. Cardiovascular pharmacology: an update. Anesthesiology Clin 2010; 28:723-38
- LOMBARD FW, GRICHNIK KP. Update on management strategies for separation from cardiopulmonary bypass. Curr Opin Anaesthesiol 2011; 24:49-57
- LOULMET DF, YAFFEE DW, URSOMANNO PA, et al. Systolic anterior motion of the mitral valve: a 30-year perspective. J Thorac Cardiovasc Surg 2012; 143:52-7
- MASLOW AD, REGAN MM, PANZICA P, et al. Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg 2002; 95:1507-18
- McCARTNEY SL, DUCE L, GHADIMI K. Intraoperative vasoplegia: methylene blue to the rescue! Curr Opin Anesthesiol 2018; 31:43-9
- NIELSEN DV, HANSEN KM, JOHNSEN SP, et al. Health outcomes with and without use of inotropic therapy in cardiac surgery. Anesthesiology 2014; 120:1098-108
- NOVITZKY D, FONTANET H, SNYDER M, et al. Impact of tri-iodo-thyronine on the survival of high-risk patients undergoing heart surgery. Cardiology 1996; 87:509-15
- PAGEL PS. Levosimendan in cardiac surgery: A unique drug for the treatment of perioperative left ventricular dysfunction or just another inodilator searching for a clinical application? Anesth Analg 2007; 104:759-61
- PAPDOPOULOS G, SINTOU E, SIMINELAKIS S, et al. Perioperative infusion of low-dose of vasopressin for prevention and management of vasodilatory vasoplegic syndrome in patients undergoing coronary artery bypass grafting - a double-blind randomized study. J Cardiothorac Surg 2010; 5:17
- PATEL BM, CHITTOK DR, RUSSEL JA, WALLEY KR. Beneficial effects of short-term vasopressin infusion during severe septic shock. Anesthesiology 2002; 96:576-82
- PISANO A, MONTI G, LANDONI G. Levosimendan: new indications and evidence for reduction in perioperative mortality? Curr Opin Anaesthesiol 2016; 29:454-61
- POLLESELLO P, PARISSIS J, KIVIKKO M, et al. Levosimendan meta-analyses: is there a pattern in the effect on mortality? Int J Cardiol 2016; 209: 77-83
- ROYSTER RL. Myocrdial Dysfunction following Cardiopulmonary Bypass: Recovery patterns, predictors of initropic needs, theoretical concepts of inotropic administration. J Cardiothor Vasc Anesth 1993; 7 (Suppl 2): 19-25
- SCHWINN DA, LEONE BJ, SPAHN DR, et al. Desensitization of myocardial b-adrenergic receptors during cardiopulmonary bypass. Circulation 1991; 84:2559-67
- SHAEFI S, MITTEL A, KLICK J, et al. Vasoplegia after cardiovascular procedures - Pathophysiology and targeted therapy. J Cardiothorac Vasc Anesth 2018; 32:1013-22
- SHI Y, DENAULT AY, COUTURE P, et al. Biventricular filling patterns after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2006; 131:1080-6
- SHIM JK, YANG SY, YOO YC, et al. Myocardial protection by glucose-insulin-potassium in acute coronary syndrome patients undergoing urgent multivessel off-pump coronary artery bypass surgery. Br J Anaesth 2013; 110:47-53
- SMILEY RM, VUILLEMOZ Y. Cardiac surgery causes desensitization of the b-adrenergic system of immune lymphocytes. Anesth Analg 1992; 74:212-8
- TOLLER W, HERINGLAKE M, GUARRACINO F, et al. Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int J Cardiol 2015; 184:323-36
- VAN WEZEL HB, DEJONG SWM. Clinical use of glucose-insulin-potassium in cardiac surgery and acute myocardial infarction: an overview. Semin Cardiothorac Vasc Anesth 2003; 7:77-83
- VITEZ TS. Pro: Calcium salts are contraindicated in the weaning of patients from cardiopulmonary bypass J Cardiothor Vasc Anesth 1988; 2:567-70
- VROOM MB. Epidemiology and pharmacotherapy of acute heart failure. Semin Cardiothorac Vasc Anesth 2003; 7:3-12
- WEINER MM, LIN HM, DANFORTH D, et al. Methylene blue is associated with poor outcomes in vasoplegic shock. J Cardiothorac Vasc Anesth 2013; 27:1233-8
- WOODS KL, FLETCHER S. Long-term outcome after intravenous magnesium sulphate in suspected acute myocardial infarction: The second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2). Lancet 1994; 343:816-9