Aortic valve repair
Aortic valve repair is an option that is increasingly being used in specialist centres for aortic insufficiency due to cusp prolapse or annular dilatation caused by aortic root disease. The aim is to restore the anatomy and function of all components of the aortic root. The TEE examination prior to ECC must assess the feasibility of the procedure and the mechanism of valvular incompetence. By analogy with the classification of MI, 3 types of AI are described, which define the type of reconstruction possible (Figure 11.59) [3,8,19,26].
- Type I: Normal cusp movement, dilatation of the aortic root; central jet.
- Type IA: Dilatation of the sinotubular junction;
- Type IB: Dilatation of the sinuses of Valsalva, obliteration of the sino-tubular junction, widening of the commissures preventing coaptation of the cusps;
- Type IC: dilatation of the aortic annulus;
- Type ID: cusp perforation (variable jet).
- Type II: excess tissue and movement of one or more cusps, prolapse; eccentric jet.
- Type III: restrictive movement of the cusps, retraction, fibrosis, calcification; variable jet.
In type I, the type of repair varies according to the degree of anatomical damage.
- Type IA: Replacement of the ascending aorta with a Dacron tube and remodelling of the sino-tubular junction, resuspending the cusps (see Figure 11.143).
- Type IB: Replacement of the sinuses of Valsalva, sparing the valve which is reimplanted in the prosthesis, reimplantation of the coronaries (see Figure 11.144) [5].
- Type IC: Subcommissural annuloplasty (sutures or Teflon tape), internal annuloplasty.
- Type ID: patching perforation with pericardium.
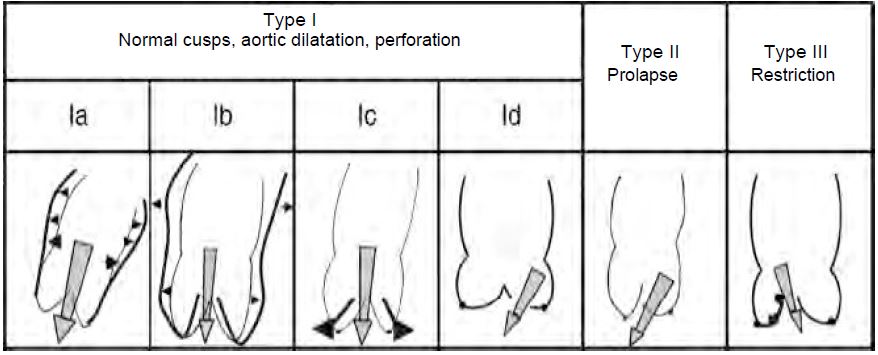
Figure 11.59: The different anatomopathological types of aortic insufficiency, classified according to the degree of mobility of the cusps, with an illustration of the mechanisms involved [8].
In type II, the prolapsing part of the cusps is resected and sutured or plicated, the free edges are resuspended by a suture at the level of each commissure; the procedure usually involves annuloplasty at the level of the annulus and/or the sinotubular junction [4,26]. Type III is more difficult and requires decalcification and reconstruction of the cusps with a patch of pericardium or a homologous valve. The failure rate for aortic repair is higher than for mitral repair (14% versus 6%) [9,14], and the risk of complications at 10 years is 12% [1]; the risk of revision at 5 years for recurrent AI is 11% [15]. However, operative mortality is lower than for valve replacement: < 1% for isolated cusp repair and 3.4% for aortic root repair [1]. Plasty is not more difficult in bicuspid patients unless the cusps are fibrotic, calcified or thickened at the commissures [16], but the indication is important because the patients are younger.
Video: Prolapse of the anterior cusp in a case of aortic bicuspidism, leading to severe regurgitation.
Video: Image of colour flow regurgitation in the same case.
Video: Long-axis view of the aortic plasty in the previous case; no residual leakage, satisfactory cusp contact in diastole.
After correction, the TEE examination is used to decide whether the repair was successful or whether further surgery is required. A good result is indicated by (Figure 11.60) [13]:
- Coaptation of the cusps above the plane of the aortic annulus in the 120° long axis view, but not below; no prolapse in the LVOT.
- Height of coaptation zone > 0.4 cm.
- Cusp height > 0.8 cm (distance between the highest point of the coaptation and the point where the cusps are implanted on the ring); ideally, the highest point should be at mid-height of the sinuses of Valsalva.
- Free movement of cusps in systole, cusps parallel to flow.
- Aortic insufficiency < mild.
- Systolic ΔPmax < 30 mmHg, ΔPmoy ≤ 15 mmHg.
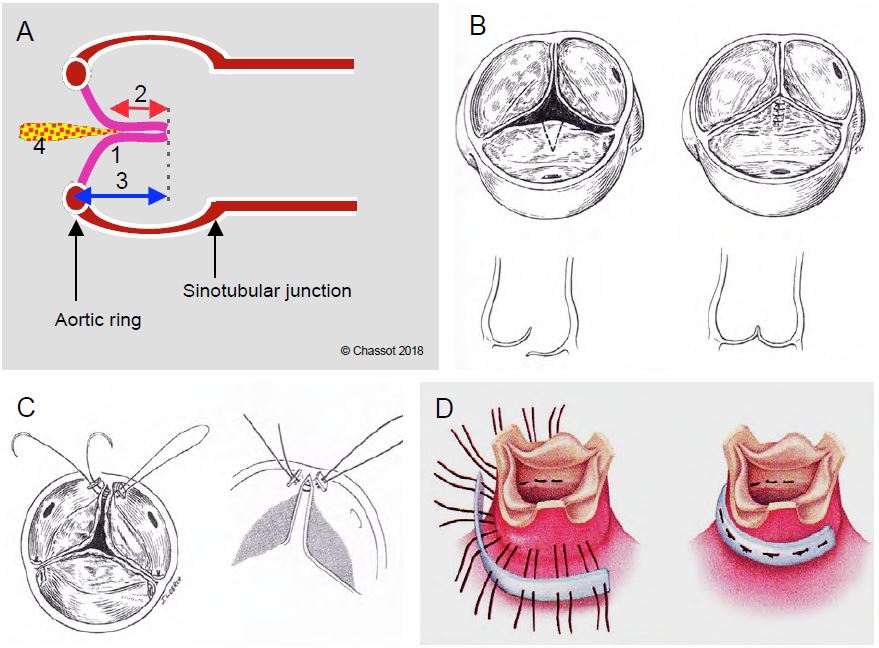
Figure 11.60: Aortic grafting. A: Diagram of the conditions for successful plasty; no prolapse, coaptation below (above) the plane of the aortic annulus (1), coaptation height > 4 mm (2, red arrow), cusp height > 8 mm (3, blue arrow), residual AI < minor (4). B: Triangular resection of a unicuspid prolapse and suture of the cusp. C: Resuspension of a commissure. D: Aortic annuloplasty [Braunwald's Heart Disease, 7th ed, Philadelphia:Elsevier 2005, 1601].
The following elements are considered to be a failure of the procedure and require a return to ECC for repair or valve replacement [13].
- Residual AI more than insignificant;
- Level of coaptation of the cusps below the plane of the aortic ring;
- Height of coaptation of the free edge of the cusps ≤ 0.4 cm;
- Excessive gradient: ΔPmax > 30 mmHg, ΔPmoy > 15 mmHg.
| Aortic valve replacement (AVR) |
|
Valvuloplasty, which can be performed in 60% of cases of aortic insufficiency, is always preferable to replacement because it restores normal anatomy. Advantages over prosthesis:
- No anticoagulation
- Very low mechanical and infectious risks
- Three times lower operative mortality (1-2%); for aortic root replacement: 3-4%.
- Redo op after 10 years: 12%
AVR involves repair of the aortic root, external annuloplasty, resection and resuspension of the cusps.
Feasibility criteria for aortic repair:
- Dilatation of the aortic ring or root but normal cusps
- Prolapse of one or more cusps
- No stenosis or calcification
- Bicuspid valve is not a contraindication
Aortic graft success criteria (post-ECC examination, average failure rate 14%) :
- No or minimal residual AI (MAP > 80 mmHg, satisfactory LV function)
- Adequate confrontation of the lamellae behind the ring (coaptation height > 4 mm)
No prolapse, cusp height > 8 mm
- Mean anterograde gradient ≤ 10 mmHg
|
During coronary reimplantation, it is important to check for the presence of flow in the roots of both trunks using colour Doppler and to look for any alteration in segmental kinetics that may indicate truncal ischaemia.
Tricuspidoplasty
The presence of moderate to severe residual tachycardia is detrimental to the prognosis of any cardiac procedure and tends to worsen subsequently despite correction of left-sided valvular disease [17,22]. As a result, the indications for tricuspid repair are broad and are often based on intraoperative TEE (see Tricuspid pathology, Indications and surgical results) [2,7,18,21,24,25].
- Severe symptomatic TI of organic or functional origin that does not respond to medical treatment;
- Severe primary TI, even if asymptomatic, with dilatation of the RV and signs of progressive right-sided dysfunction;
- Severe primary or secondary TI in patients requiring left-sided valve surgery;
- Moderate or severe TI with significant annular dilatation (diameter > 4.0 cm in 4 cavities or > 7.0 cm in the surgical field) in patients requiring left heart surgery;
- Severe traumatic TI, even if asymptomatic (not in emergency).
Several correction techniques are available to the operator (Figure 11.61 and Figure 11.159) [24].
Video: Tricuspid plasty of a traumatic rupture of the anterior leaflet chords; no residual leak in systole and no stenosing effect in diastole (no colour flow aliasing).
- Annuloplasty using narrowing sutures along the insertion of the anterior and posterior leaflets (DeVega plasty).
- Plication of the annulus in its posterior part, completely obliterating the posterior leaflet and transforming the tricuspid valve into a bicuspid valve (Kay's bicuspidisation).
- Plasty by insertion of a semi-rigid ring (Carpentier) or a saddle-shaped ring (MC3); this ring is interrupted at the level of the septum to avoid damage to the atrioventricular node and the His bundle.
- Common suture of the ends of the 3 leaflets, transforming the valve into a three-leaf clover shape in diastole (similar to the Alfieri technique for the mitral valve).
- Reimplantation of chords in the event of rupture; resection of prolapse.
- In patients with conduction block, an epicardial lead is implanted at the same time as the tricuspid valve prosthesis, as it is not possible or advisable to insert a pacemaker lead through the epicardium.
- In the event of failure: replacement of the valve with a prosthesis. A bioprosthesis is implanted instead of a mechanical prosthesis because their survival is identical. In fact, the right heart is less stressed than the left, so bioprostheses have a longer life in the tricuspid position than in the mitral or aortic position [6,11]. On the other hand, mechanical prostheses have a much higher rate of thrombosis because the flow is at a very low pressure.
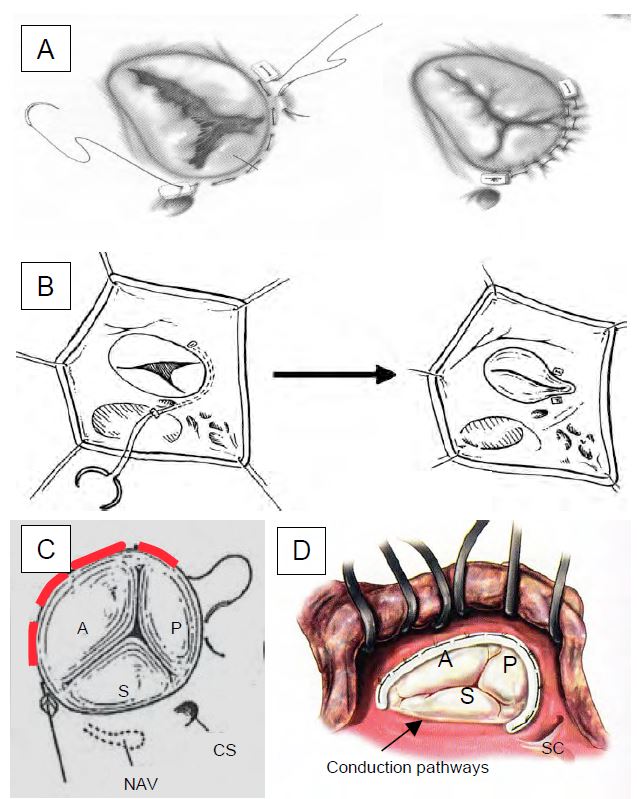
Figure 11.61: Tricuspidoplasty seen through an atriotomy of the RA as it appears in the operative field.
A: Posterior annuloplasty.
B: Bicuspidalisation according to Kay.
C: De Vega plasty; sliding points are placed on the ring from the anteroseptal commissure to the posteroseptal commissure; they allow the ring to be narrowed at will by calibrating it with a 27 gauge [20].
D: Tricuspid prosthetic ring; it is interrupted at the level of the septum to avoid damaging the conduction pathways during insertion [Savage RM, ed. Intraoperative transesophageal echocardiography. Philadelphia: Lippincott Williams & Wilkins, 2005, 440]. NAV: Node of the atrioventricular system. SC: coronary sinus. A: anterior leaflet. P: Posterior leaflet. S: septal leaflet.
After correction, the mean gradient across the tricuspid valve must remain < 3 mmHg [10]. The average failure rate is 14% [20]. Functional results are better (85% of cases without revision at 10 years) and survival at 15 years is doubled with prosthetic annuloplasty compared to simple plasty according to De Vega [23]. The conditions for successful tricuspidoplasty are [12,23]:
- Small residual TI;
- Pmean < 3 mmHg;
- Vena contracta < 0.3 cm;
- Absence of PISA and systolic reflux in the IVC and suprahepatic veins.
Prosthetic valve replacement is always preferable to surgical valve replacement because of the inferior results of the latter: higher rate of postoperative right-sided failure (28% versus 9%), increased mortality (11% versus 4-7%), anticoagulation, risk of thrombosis (1%/year) [22,23,27]. The prosthesis is a rigid mechanical element that blocks the circular contraction of the base of the RV and limits its longitudinal contraction [20]. Right ventricular dysfunction is the most important determinant of postoperative mortality, more important than pulmonary hypertension [21].
| Tricuspid valve repair (TVR) |
| In the case of secondary TI, TVR consists of restrictive suturing of the ring (De Vega) or prosthetic ring placement. In primary TI: resection as needed, insertion of a ring.
Indications for TVR:
- Severe symptomatic TI (right-sided failure)
- Moderate to severeTI secondary to surgically corrected left-sided valve disease
- Moderate to severe TI if ring dilatation (> 2.8 cm in 4-cavity view) during left heart surgery
- Severe traumatic TI, even if asymptomatic (not in emergency)
|
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- AICHER D, FRIES R, RODIONYCHEVA S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010; 37:127-32
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BOODWHANI MJ, DE KERCHOVE L, GLINEUR D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009; 137:286-94
- COSGROVE DM, ROSENKRANZ ER, HENDREN WG, et al. Valvuloplasty for aortic insufficiency. J Thorac Cardiovasc Surg 1991; 102:571-7
- DAVID TE. Aortic valve sparing in different aortic valve and aortic root conditions. J Am Coll Cardiol 2016; 68:654-64
- DEL CAMPO C, SHERMAN JR. Tricuspid valve replacement: Results conmparing mechanical and biological prostheses. Ann Thorac Surg 2000; 69:1295-303
- DREYFUS GD, CORBI PJ, CHAN KM, et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann THorac Surg 2005; 79:127-32
- EL KHOURY G, GLINEUR D, RUBAY J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Cur Opin Cardiol 2005; 20:115-21
- GRIMM RA, STEWART WJ. The role of intraoperative echocardiography in valve surgery. Cardiol Clin 1998; 16:477-89
- HAHN RT. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging 2016; 9:e005332
- KUNADIAN B, VIJAYALAKSHMI K, BALASUBRAMANIAN S, et al. Should the tricuspid valve be replaced with a mechanical or biological valve? Interact Cardiovasc Thorac Surg 2007; 6:551-7
- K, MORISHITA K, TSUKAMOTO M, et al. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur J Cardiothorac Surg 2001; 20:577-8
- LE POLAIN DE WAROUX JB, POULEUR AC, ROBERT A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair. Predictive value of transesophageal echocardiography. JACC Cardiovasc Imaging 2009; 2:931-9
- MAZZUCOTELLI JP, DELEUZE PH, BEAUFRETON C, et al. Preservation of the aortic valve in acute aortic dissection: long-term echocardiographic assessment and clinical outcome. Ann Thorac Surg 1993; 55.1513-17
- MINAKATA K, SCHAFF HV, ZEHR KJ, et al. Is repair of aortic valve regurgitation a safe alternative to valve replacement? J Thorac Cardiovasc Surg 2004; 127:645-53
- NASH PJ, VITVITSKY E, LI J, et al. Feasibility of valve repair for regurgitant bicuspid aortic valves - An echocardiographic study. Ann Thorac Surg 2005; 79:1473-9
- NATH J, FOSTER E, HEIDENREICH PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004; 43:405-9
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- PRODROMO J, D'ANCONNA G, AMADUCCI A, et al. Aortic valve repair fort aortic insufficiency: a review. J Cardiothorac Vasc Anesth 2012; 26:923-32
- ROGERS JH, BOLLING SF. The tricuspid valve. Current perspective and evolving management of tricuspid regurgitation. Circulation 2009; 119:2718-25
- SHIRAN A, SAGIE A. Tricuspid regurgitation in mitral valve disease. J Am Coll Cardiol 2009; 53:401-8
- SINGH SK, TANG GH, MAGANTI MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg 2006; 82:1735-41
- TANG GH, DAVID TE, SINGH SK, et al Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation 2006; 114(suppl I):577-81
- TARAMASSO M, VANERMEN H, MAISANO F,et al. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol 2012; 59:703-10
- VAHANIAN A, ALFIERI O, ANDREOTTI F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012; 33:2451-96
- VAN DYCK MJ, WATREMEZ C, BOODHWANI M, et al. Transesophageal echocardiography evaluation during aortic valve repair surgery. Anesth Analg 2010; 111:59-70
- ZACK CJ, FENDER EA, CHANDRARASHEKAR P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol 2017; 70:2953-60
