even percent of paediatric congenital anomalies are ASDs. They are the second most common form of congenital heart disease after VSDs. Five types of defects may occur in the atrial septum (Figure 14.36) [5].
- Ostium secundum situated in the centre of the septum at the site of the fossa ovalis, accounting for 75% of cases;
- Ostium primum: defect situated near the tricuspid valve, which forms is part of the atrioventricular canal defect (15% of cases);
- Sinus venosus situated at the origin of a vena cava (usually the SVC), often associated with partial anomalous pulmonary venous return (5-10% of cases);
- Absence of the coronary sinus roof allowing it to communicate with the LA (< 1%);
- Special case: patent foramen ovale (PFO).
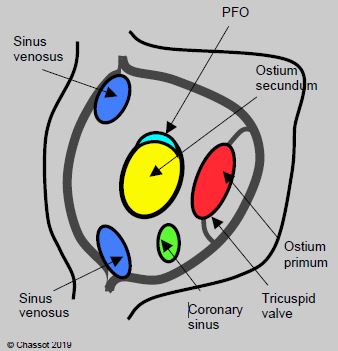
Figure 14.36: View of the atrial septum from the RA with the positioning of the various anatomical variants of atrial septal defects [3].
The flow rate of L-to-R shunting is dependent on the size of the ASD and the respective compliance of the right and left chambers: if LV compliance is reduced, LAP rises and L-to-R shunting increases, since the shunt’s flow is mostly diastolic. It causes volume overload on the right side and therefore dilation of the RA, RV and PA (Figure 14.37).
The RV is dilated and hypertrophied to accommodate the volume overload (Video).
The PA diameter is larger than that of the aorta and the flow is accelerated (see Figure 15.11). The normal ratio of 0.6 between the right and left dimensions and between PA and aorta maximal flow velocity is more than doubled. If it occurs, pulmonary hypertension is a delayed phenomenon (> 20 years) – PVR rarely exceeds 500 dynes•s•cm-5. Unlike VSDs, ASDs do not transmit systemic arterial pressure to the pulmonary system.
The flow through the shunt exhibits a typical biphasic pattern (Vmax 0.5-1.5 m/s), with an end systolic - early diastolic peak corresponding to the v-wave and a peak during atrial contraction corresponding to the a-wave (Video).
Video: Colour flow of the L-to-R shunt between LA (above) and RA (below).
Between them, there are two periods during which the flow rate slows or even reverses – these correspond tothe pressure drops “x” and “y” (Figure 14.38) [9].
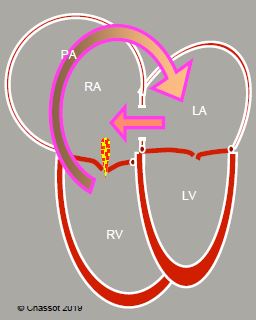
Figure 14.37: Atrial septal defect. Diagram showing the outline of the 4 cardiac chambers in the event of ASD with major left → right shunting (arrow). The RA and RV are dilated. The RV is hypertrophied. The apex of the heart is formed by the RV and not the LV. RV dilation results secondarily in tricuspid insufficiency (TI).
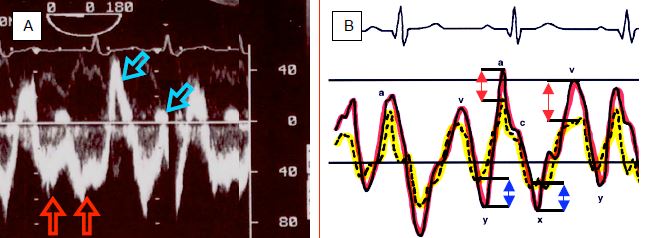
Figure 14.38: Spectral Doppler image of the flow through an ASD. A: the shunt is predominantly left-to-right (flow below the baseline, moving away from the transducer located in the oesophagus), with two components (red arrows). It also exhibits two minor right-to-left components above the baseline (blue arrows). B: representation of the pressure curves of the LA (red) and RA (yellow). Pressure variations are more significant on the left side. Consequently, PLA is higher than PRA during pressure peaks, but lower during pressure troughs. The flow is therefore left-to-right during the a- and v-waves (red arrows), but right-to-left during the x and y drops (blue arrows) [8,9].
This morphology relates to pressure variations in the atria during the cardiac cycle [8]. Indeed, the amplitude of pressure variations is greater in the LA than in the RA – pressure is therefore higher on the left side than on the right side during pressure peaks, but lower during pressure troughs, enabling the flow to reverse at this moment. The most significant inversion occurs during systole since the descent of the mitral annulus causes a sudden increase in LA volume and a major fall in its pressure. The shunt's R-to-L component intensifies if RA filling increases due to a drop in intrathoracic pressure (relaxation post-Valsalva or Müller's manoeuvre) – the same applies if RAP increases due to rising RV afterload and filling pressure e.g. during inspiration of IPPV or with PEEP (Figure 14.39) [7].
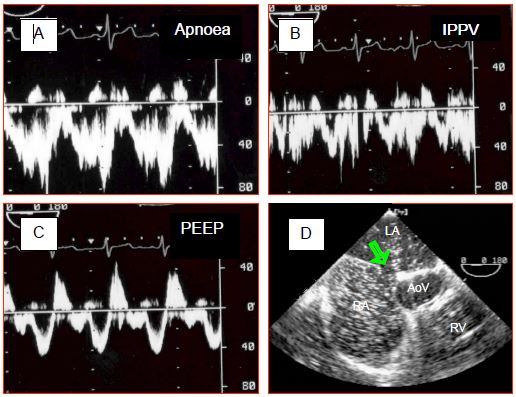
Figure 14.39: Impact of ventilation on ASD flow. A: During apnoea, the flow is virtually exclusively left-to-right. B: During IPPV, the velocity of the left-to-right flow decreases. C: Adding PEEP 10 cm H2O further reduces the left-to-right component and increases the right-to-left component. Although the lesion is not cyanotic, arterial saturation falls. D: Paradoxical embolism may occur at times when RAP is higher than LAP, as indicated by the movement of microbubbles from the RA to the LA in the presence of PEEP (green arrow: ASD).
Therefore, even a predominantly L-to-R shunt may cause paradoxical embolism as a result of a Valsalva manoeuvre or excessive PEEP. This requires the anaesthetist to “hunt for bubbles” in all venous lines to avoid cerebral or coronary gas embolism.
Small ASDs may remain asymptomatic for a very long time, the only sign being a high-flow grade 3/6 systolic murmur through the pulmonary valve that can be heard at the upper left edge of the sternum, and a split second heart sound. ASDs < 5 mm without any signs of right-side overload or PHT do not require surgery. ASDs associated with an increase in pulmonary blood flow of over 50% (Qp: Qs > 1.5), paradoxical embolism, and right-side dilation must be closed to prevent arrhythmias (atrial tachyarrhythmias) and right ventricular decompensation. No sequelae occur if the operation is performed before the age of 4-5 years, while right ventricular hypertrophy and RA dilation persist if correction takes place after the age of 5 years [6]. If PHT is present, closure of an ASD is only indicated if the shunt is mostly L-to-R and if the PVR/SVR ratio is < 0.3 [2].
Central ASDs (ostium secundum or PFO) of dimension under 40 mm and surrounded by appropriately-sized edges (> 5 mm) can be occluded by a prosthesis, which is fitted percutaneously (Amplatzer™, PFO Star™, Helex™ occluders, etc.). This treatment can only be used on children > 25 kg with Qp/Qs > 1.5 but no PHT [11]. The main complications are arrhythmias, conduction disorders, tamponade, embolisation (0.55%), and erosion (0.1%). Erosion is linked to the narrowness of the septal edge in which the prosthesis is implanted, especially in the anterosuperior area, where the prosthesis may erode the nearby aorta [1]. Although the incidence of such complications is < 1%, long-term outcomes are satisfactory [4]. Large ASDs, ASDs of a different type than ostium secundum and PFO, or ASD combined with other cardiac malformations, are closed surgically (autologous pericardial patch or direct closure), by sternotomy or by right-side mini-thoracotomy. The surgical mortality rate is < 1%. In both approaches, transesophageal echocardiography is very useful for guiding the closing device and checking leak tightness after surgery.
Anaesthesia
Children with a simple ASD are operated on around the age of 3-5 years. They are generally in good health and tolerate all anaesthetic techniques – they may be extubated at the end of the operation or within an hour post-surgery.
Between them, there are two periods during which the flow rate slows or even reverses – these correspond tothe pressure drops “x” and “y” (Figure 14.38) [9].

Figure 14.37: Atrial septal defect. Diagram showing the outline of the 4 cardiac chambers in the event of ASD with major left → right shunting (arrow). The RA and RV are dilated. The RV is hypertrophied. The apex of the heart is formed by the RV and not the LV. RV dilation results secondarily in tricuspid insufficiency (TI).

Figure 14.38: Spectral Doppler image of the flow through an ASD. A: the shunt is predominantly left-to-right (flow below the baseline, moving away from the transducer located in the oesophagus), with two components (red arrows). It also exhibits two minor right-to-left components above the baseline (blue arrows). B: representation of the pressure curves of the LA (red) and RA (yellow). Pressure variations are more significant on the left side. Consequently, PLA is higher than PRA during pressure peaks, but lower during pressure troughs. The flow is therefore left-to-right during the a- and v-waves (red arrows), but right-to-left during the x and y drops (blue arrows) [8,9].
This morphology relates to pressure variations in the atria during the cardiac cycle [8]. Indeed, the amplitude of pressure variations is greater in the LA than in the RA – pressure is therefore higher on the left side than on the right side during pressure peaks, but lower during pressure troughs, enabling the flow to reverse at this moment. The most significant inversion occurs during systole since the descent of the mitral annulus causes a sudden increase in LA volume and a major fall in its pressure. The shunt's R-to-L component intensifies if RA filling increases due to a drop in intrathoracic pressure (relaxation post-Valsalva or Müller's manoeuvre) – the same applies if RAP increases due to rising RV afterload and filling pressure e.g. during inspiration of IPPV or with PEEP (Figure 14.39) [7].

Figure 14.39: Impact of ventilation on ASD flow. A: During apnoea, the flow is virtually exclusively left-to-right. B: During IPPV, the velocity of the left-to-right flow decreases. C: Adding PEEP 10 cm H2O further reduces the left-to-right component and increases the right-to-left component. Although the lesion is not cyanotic, arterial saturation falls. D: Paradoxical embolism may occur at times when RAP is higher than LAP, as indicated by the movement of microbubbles from the RA to the LA in the presence of PEEP (green arrow: ASD).
Therefore, even a predominantly L-to-R shunt may cause paradoxical embolism as a result of a Valsalva manoeuvre or excessive PEEP. This requires the anaesthetist to “hunt for bubbles” in all venous lines to avoid cerebral or coronary gas embolism.
Small ASDs may remain asymptomatic for a very long time, the only sign being a high-flow grade 3/6 systolic murmur through the pulmonary valve that can be heard at the upper left edge of the sternum, and a split second heart sound. ASDs < 5 mm without any signs of right-side overload or PHT do not require surgery. ASDs associated with an increase in pulmonary blood flow of over 50% (Qp: Qs > 1.5), paradoxical embolism, and right-side dilation must be closed to prevent arrhythmias (atrial tachyarrhythmias) and right ventricular decompensation. No sequelae occur if the operation is performed before the age of 4-5 years, while right ventricular hypertrophy and RA dilation persist if correction takes place after the age of 5 years [6]. If PHT is present, closure of an ASD is only indicated if the shunt is mostly L-to-R and if the PVR/SVR ratio is < 0.3 [2].
Central ASDs (ostium secundum or PFO) of dimension under 40 mm and surrounded by appropriately-sized edges (> 5 mm) can be occluded by a prosthesis, which is fitted percutaneously (Amplatzer™, PFO Star™, Helex™ occluders, etc.). This treatment can only be used on children > 25 kg with Qp/Qs > 1.5 but no PHT [11]. The main complications are arrhythmias, conduction disorders, tamponade, embolisation (0.55%), and erosion (0.1%). Erosion is linked to the narrowness of the septal edge in which the prosthesis is implanted, especially in the anterosuperior area, where the prosthesis may erode the nearby aorta [1]. Although the incidence of such complications is < 1%, long-term outcomes are satisfactory [4]. Large ASDs, ASDs of a different type than ostium secundum and PFO, or ASD combined with other cardiac malformations, are closed surgically (autologous pericardial patch or direct closure), by sternotomy or by right-side mini-thoracotomy. The surgical mortality rate is < 1%. In both approaches, transesophageal echocardiography is very useful for guiding the closing device and checking leak tightness after surgery.
Anaesthesia
Children with a simple ASD are operated on around the age of 3-5 years. They are generally in good health and tolerate all anaesthetic techniques – they may be extubated at the end of the operation or within an hour post-surgery.
- Preferred anaesthetic technique: induction by inhaled agent (sevoflurane) or intravenous agent (propofol, etomidate, ketamine). Maintenance with sevoflurane (1-2%), fentanyl
- (10-15 mcg/kg), rocuronium (0.6-1.2 mg/kg). In young children, caudal anaesthesia with morphine (70 mcg/kg in 5-10 mL NaCl 0.9% preservative-free) post-induction provides excellent postoperative analgesia [10].
- Haemodynamic management is aimed at reducing the extent of shunting. This is achieved by lowering systemic vascular resistance (SVR) and slightly increasing pulmonary vascular resistance (PVR) (see Figure 14.8). Provided that PHT is not present, this is achieved by systemic vasodilation and slight hypoventilation (FiO2 0.3, PaCO2 45 mmHg).
- Positive pressure ventilation (IPPV) and particularly PEEP raise the RV afterload, impeding the shunt's L-to-R component while increasing the R-to-L component. In patients with partially bidirectional shunting, it may cause arterial desaturation. Therefore, it is advised to ventilate these patients at low pressure.
- Any rise in pressure in the RA increases the risk of paradoxical embolism – this may occur following peripheral thromboembolism or accidental injection of air or particles through venous lines. Any air bubbles must be meticulously eliminated from infusions and bubble filters must be used on slow infusion lines. Unfortunately, it is not possible to administer propofol, etomidate or rapid volume through these filters.
- In the event of pulmonary arterial hypertension (PAH), positive pressure ventilation further increases right-sided afterload. However, this rise in pressure is small compared to resting pulmonary arterial pressure. It represents an even smaller increase in afterload than in normal individuals. Moreover, with controlled ventilation it is possible to lower PVR by hyperventilation and hypocapnia (see Pulmonary Hypertension). The pulmonary vascular musculature is hyperreactive in the postoperative phase.
- Systemic arterial vasodilation reduces left-side pressure and lowers the volume of the L-to-R shunt. From this perspective, isoflurane and spinal regional anaesthesia are appropriate choices. However, if the shunt is already bidirectional because right-sided overload has induced PAH, the shunt’s R-to-L component increases along with arterial desaturation.
- Hypovolaemia is poorly tolerated, since the patient needs high circulating volume to maintain his systemic flow, given that some of the volume is inevitably sequestrated by the shunt between the RA and the LA via the low-pressure pulmonary circuit. This is a particularly significant issue if SVR is high.
- Measurement of CVP is a poor indicator of preload, since RAP is high due to the shunt and any tricuspid insufficiency.
- The RV requires a particularly high preload if it is hypertrophied. Right ventricular function may be improved as required by increasing contractility and heart rate (dobutamine, milrinone).
- Closing an ASD renders the patient suddenly hypervolaemic, since the volume previously diverted to the pulmonary circuit by the shunt, which had only circulated between the RA and LA, is added to the systemic circulating volume.
- In case of an ASD closure by right-sided thoracotomy, postoperative analgesia may be performed in two ways: either by an intercostal block in the form of a surgeon-performed infiltration of bupivacaine 0.5% (2.5 mg/kg) or ropivacaine 0.2% (3 mg/kg, 2 mg/kg < 1 year), or by a single-shot thoracic paravertebral block performed by the anaesthetist post-induction or at the end of surgery (pre-extubation) [12].
The most common complications are atrial arrhythmias (proportionate to RA dilation), complete AV block (especially in cases of ostium primum), defects with patches (residual shunting), and mitral insufficiency in cases of ostium primum. Although common, a highly pathological ECG with major ST segment elevation in all derivations is not clinically significant.
| Atrial septal defects (ASDs) |
|
Characteristics:
- Non-cyanotic L-to-R shunt with increased pulmonary blood flow (Qp ↑, Qp:Qs > 1.5 :1) - Possible small R-to-L component (risk ↑ if PAH or ↑ RAP) - Dilation of receiving chambers RA, RV, PA - Volume overload for the RV - Pulmonary hypertension uncommon (< 10% of adults: PVR 300-500 dynes•cm•s-5) - Surgical indications: Qp/Qs > 1.5, RV dilation, exertional dyspnoea Management: reduce shunting by lowering SVR and increasing PVR Anaesthesia recommendations: - Vasodilatation with isoflurane (or sevoflurane) - Ventilation: FiO2 0.3, normocapnia or slight hypercapnia, low ventilation P, no PEEP - Hypovolaemia poorly tolerated due to the sequestration of volume in the pulmonary circulation - Prevent any bubbles from entering iv tubings (risk of R-to-L crossing), use bubble filters - Rapid extubation |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- AMIN Z, HIJAZI ZM, BASS JL, et al. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects : review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv 2004 ; 63 :496-502
- BENT ST. Anesthesia for left-to-right shunt lesions. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 297-327
- BETTEX D, CHASSOT PG. Transesophageal echocardiography in congenital heart disease. In: BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1186-1212
- CHESSA M, CARMINATI M, BUTERA G, et al. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 2002; 39:1061-5
- DANIELS SR. Epidemiology. In: LONG WA. Fetal and neonatal cardiology. Philadelphia: WB Saunders, 1990, 430
- DAVLOUROS PA, NIWA K, WEBB G, GATZOULIS MA. The right ventricle in congenital heart disease. Heart 2006; 92(Suppl 1): i27-i38
- JAFFE RA, PINTO FJ, SCHNITTGER I, et al. Aspects of mechanical ventilation affecting interatrial shunt flow during general anesthesia. Anesth Analg 1992; 75:484-8
- LEVIN AR. Atrial pressure-flow dynamics in atrial septal defects (secundum type). Circulation 1968; 37:476-9
- LIN FC, FU M, YEH SH, WU D. Doppler atrial flow patterns in patients with secundum atrial septal defects. Determinants, limitations and pitfalls. J Am Soc Echocardiogr 1988; 1:141-5
- NASR VG, DINARDO JA. The pediatric cardiac anesthesia handbook. Oxford: Wiley-Blackwell, 2017; 83-87
- TABIS J, SHENODA M. Percutaneous treatment of patent foramen ovale and atrial septal defects. J Am Coll Cardiol 2012; 60:1722-32
- TAHARA S, INOUE A, SAKAMOTO H, et al. A case series of continuous paravertebral block in minimally invasive cardiac surgery. JA Clin Rep 2017; 3:45
