In order to navigate among the numerous existing congenital heart diseases, it is useful to refer to the basic segmental analysis concept adopted by anatomical pathologists. This divides the heart into three segments (Figure 14.27) [6,7].
- The atrial segment, which includes the atria and the great veins;
- The ventricular segment, which includes the ventricles and atrioventricular valves;
- The arterial segment, which includes the aorta and the pulmonary artery with their respective valves.
These three segments are joined by two junctions:
- The atrioventricular junction,
- The ventriculoarterial junction.
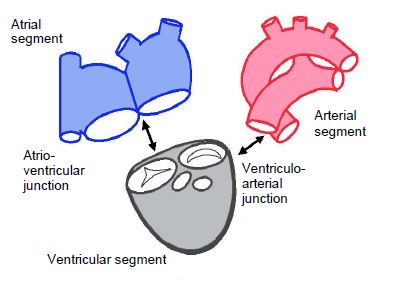
Figure 14.27: Segmental analysis. The heart is split into three segments connected by two junctions. It should be noted that the mitral and tricuspid valves belong to the ventricular segment, while the aortic and pulmonary valves are connected to the arterial segment [7].
Five criteria are used to determine the heart's anatomical structures [3].
- Situs – this may be solitus (normal) or inversus (heterotaxy) – since the heart's situs is consistent with abdominal situs except in very rare cases, it is essentially defined by the position of the right atrium (RA), which receives the inferior vena cava (IVC) or, in absence of IVC, the hepatic veins from the liver. The incidence of heterotaxy is 0.8 per 10,000 births [4].
- Concordance or discordance of two successive segments – instead of succeeding each other in a normal position (concordance), two segments may be in an inappropriate relative position (discordance), with the left-right axis of one at 180° to that of the other.
- Segmental connections – the junctions between segments are the atrioventricular canal between the atria and ventricles and the infundibulum between the ventricles and arteries.
- The specific markers of each cardiac chamber – for example, the LV is defined by the presence of two papillary muscles, a bicuspid mitral valve, fine trabeculation, and a partially fibrous outflow tract.
- Associated anomalies – these include dysmorphic structures, obstructive lesions, valvular disease and defects in a septum (shunting).
Each segment is defined by its intrinsic morphology, because the position and size of the cardiac cavities do not allow them to be identified for sure: each can be swapped, atrophic, hypertrophied, absent or on the wrong side. The anatomical descriptions of structures and pathologies in this section will be based mainly on echocardiographic imaging and illustrated with diagrams and images obtained by transesophageal echocardiography (TEE) [2].
Atrial segment
The cardiac situs is determined by the position of the atria. In the vast majority of cases, it is identical to the abdominal situs, with transdiaphragmatic discordances occurring only in severe and very rare pathologies. The RA can be identified by the junction with the inferior vena cava (IVC) – if the IVC is absent, the hepatic veins flow into the RA. The RA and the liver, which are both easy to identify, are therefore on the same side. The pulmonary veins may anastomose elsewhere than into the left atrium (LA) in the event of anomalous pulmonary venous return.
The atria may also be distinguished by their specific anatomy. The RA has a short, obtuse and pyramidal atrial appendage. It is the site of the fossa ovalis of the atrial septum and receives the insertion of the Eustachian valve. Its wall is partially trabeculated. The boundary between the smooth part and the trabeculated part is marked by a crest (crista terminalis), which runs from one vena cava to the other (Figure 14.28). The LA, whose wall is completely smooth, has a long, pectinate, finger-shaped atrial appendage. Its endothelial surface is smooth. The RA receives the hepatic veins via the inferior vena cava if present. The bronchi, pulmonary segments and abdominal organs exhibit characteristics consistent with atrial situs.
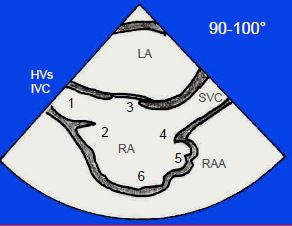
Figure 14.28: Anatomical criteria defining the RA in the 100° bicaval view. 1: inferior vena cava (IVC); if the IVC is absent, the hepatic veins (HVs) drain into the RA. 2: Eustachian membrane. 3: fossa ovalis. 4: crista terminalis. 5: atrial appendage, wide and obtuse. 6: trabeculated part. It is essential to determine which atrium is the RA as this enables to define the situs.
Sometimes both atria exhibit the same anatomical characteristics, which is described as isomerism or mirror-image atria. Right isomerism (two RAs) is normally associated with the absence of the spleen (asplenia), while left isomerism (two LAs) is characterised by multiple spleens (polysplenia) [4].
Atrioventricular junction
The junction between the atrial septum, interventricular septum, septal leaflet of the tricuspid valve and anterior leaflet of the mitral valve forms the internal crux of the heart. These four structures may exhibit a coalescence defect, leaving an orifice in the centre of the heart – this is an atrioventricular canal (AV canal) or endocardial cushion defect. Normally, the septal insertion of the tricuspid valve is 0.7 cm/m2 more distal than that of the mitral valve. Both valves are on the same plane in the event of atrioventricular canal defects (Figure 14.29) [5].
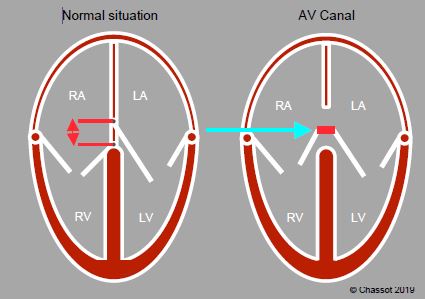
Figure 14.29: Atrioventricular junction. In normal circumstances, the septal insertion of the tricuspid valve is in a more apical position than that of the mitral valve. The difference is approximately 0.7 cm/m2. Both valves are on the same plane in cases of common atrioventricular canal defects (AV canal defects).
The atrioventricular junction is concordant if the atria and ventricles succeed each other in the correct order. It is discordant if each atrium is connected to the contralateral ventricle. In the event of discordance, the tricuspid valve remains attached to the right ventricle (RV) and the mitral valve remains attached to the left ventricle (LV). If both atria are connected to a single ventricle, this is described as a double-inlet ventricle. If an atrioventricular valve opens into both ventricles due to a ventricular septal defect (VSD), this is described as “overriding”. This malformation may be supplemented by “straddling” if the valve’s chordae tendineae are inserted into a wall of the contralateral ventricle through the VSD (Figure 14.30).
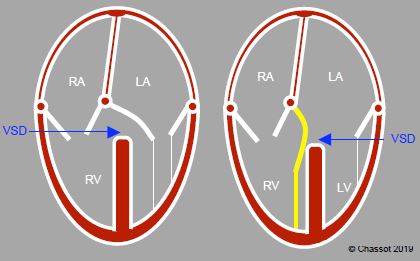
Figure 14.30: Atrioventricular junction. A: overriding – the mitral valve overrides the septum, with its orifice anastomosing to both ventricles, although the subvalvular apparatus is on the same side. It is connected to the ventricle that receives > 50% of its surface area. B: Straddling – the subvalvular apparatus of the overriding valve, in this case the mitral valve, is inserted into both sides of the septum, with part of the chordae crossing the VSD.
Ventricular segment
A ventricle is composed of three parts: an inlet, a trabeculated body, and an outflow tract. A chamber that receives more than 50% of the opening of the corresponding atrioventricular valve is considered a ventricle. Below this value, it is defined as a rudimentary chamber. Position, shape and size are unreliable indicators of which ventricle is right or left as these data may be profoundly disrupted. Other criteria are used to distinguish them (Figure 14.31) [1].
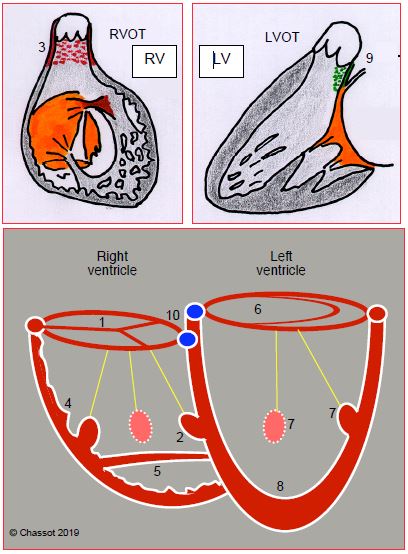
Figure 14.31: Differentiation of the RV and LV. The RV is connected to a tricuspid valve (1) and contains 3 papillary muscles, one of which is located on the septum (2); its outflow tract is entirely muscular (3); it exhibits significant trabeculation (4); and generally a clearly defined muscular moderator band (5). The LV is connected to a bicuspid valve (6) and only contains 2 papillary muscles (anterolateral and posteromedial) (7), neither of which is positioned on the septum; it is slightly trabeculated (8) and its outflow tract includes a fibrous part in the continuity between the aortic valve and anterior leaflet of the mitral valve (9). The height difference between the septal insertions of the tricuspid and mitral valves (10) helps differentiate the RV (low insertion) from the LV (high insertion) (0-20° 4-chamber view). This indicator is lost if AV canal defects are present as both valves are inserted at the same level in patients with this pathology.
A right ventricle is distinguished by:
Atrial segment
The cardiac situs is determined by the position of the atria. In the vast majority of cases, it is identical to the abdominal situs, with transdiaphragmatic discordances occurring only in severe and very rare pathologies. The RA can be identified by the junction with the inferior vena cava (IVC) – if the IVC is absent, the hepatic veins flow into the RA. The RA and the liver, which are both easy to identify, are therefore on the same side. The pulmonary veins may anastomose elsewhere than into the left atrium (LA) in the event of anomalous pulmonary venous return.
The atria may also be distinguished by their specific anatomy. The RA has a short, obtuse and pyramidal atrial appendage. It is the site of the fossa ovalis of the atrial septum and receives the insertion of the Eustachian valve. Its wall is partially trabeculated. The boundary between the smooth part and the trabeculated part is marked by a crest (crista terminalis), which runs from one vena cava to the other (Figure 14.28). The LA, whose wall is completely smooth, has a long, pectinate, finger-shaped atrial appendage. Its endothelial surface is smooth. The RA receives the hepatic veins via the inferior vena cava if present. The bronchi, pulmonary segments and abdominal organs exhibit characteristics consistent with atrial situs.

Figure 14.28: Anatomical criteria defining the RA in the 100° bicaval view. 1: inferior vena cava (IVC); if the IVC is absent, the hepatic veins (HVs) drain into the RA. 2: Eustachian membrane. 3: fossa ovalis. 4: crista terminalis. 5: atrial appendage, wide and obtuse. 6: trabeculated part. It is essential to determine which atrium is the RA as this enables to define the situs.
Sometimes both atria exhibit the same anatomical characteristics, which is described as isomerism or mirror-image atria. Right isomerism (two RAs) is normally associated with the absence of the spleen (asplenia), while left isomerism (two LAs) is characterised by multiple spleens (polysplenia) [4].
Atrioventricular junction
The junction between the atrial septum, interventricular septum, septal leaflet of the tricuspid valve and anterior leaflet of the mitral valve forms the internal crux of the heart. These four structures may exhibit a coalescence defect, leaving an orifice in the centre of the heart – this is an atrioventricular canal (AV canal) or endocardial cushion defect. Normally, the septal insertion of the tricuspid valve is 0.7 cm/m2 more distal than that of the mitral valve. Both valves are on the same plane in the event of atrioventricular canal defects (Figure 14.29) [5].

Figure 14.29: Atrioventricular junction. In normal circumstances, the septal insertion of the tricuspid valve is in a more apical position than that of the mitral valve. The difference is approximately 0.7 cm/m2. Both valves are on the same plane in cases of common atrioventricular canal defects (AV canal defects).
The atrioventricular junction is concordant if the atria and ventricles succeed each other in the correct order. It is discordant if each atrium is connected to the contralateral ventricle. In the event of discordance, the tricuspid valve remains attached to the right ventricle (RV) and the mitral valve remains attached to the left ventricle (LV). If both atria are connected to a single ventricle, this is described as a double-inlet ventricle. If an atrioventricular valve opens into both ventricles due to a ventricular septal defect (VSD), this is described as “overriding”. This malformation may be supplemented by “straddling” if the valve’s chordae tendineae are inserted into a wall of the contralateral ventricle through the VSD (Figure 14.30).

Figure 14.30: Atrioventricular junction. A: overriding – the mitral valve overrides the septum, with its orifice anastomosing to both ventricles, although the subvalvular apparatus is on the same side. It is connected to the ventricle that receives > 50% of its surface area. B: Straddling – the subvalvular apparatus of the overriding valve, in this case the mitral valve, is inserted into both sides of the septum, with part of the chordae crossing the VSD.
Ventricular segment
A ventricle is composed of three parts: an inlet, a trabeculated body, and an outflow tract. A chamber that receives more than 50% of the opening of the corresponding atrioventricular valve is considered a ventricle. Below this value, it is defined as a rudimentary chamber. Position, shape and size are unreliable indicators of which ventricle is right or left as these data may be profoundly disrupted. Other criteria are used to distinguish them (Figure 14.31) [1].

Figure 14.31: Differentiation of the RV and LV. The RV is connected to a tricuspid valve (1) and contains 3 papillary muscles, one of which is located on the septum (2); its outflow tract is entirely muscular (3); it exhibits significant trabeculation (4); and generally a clearly defined muscular moderator band (5). The LV is connected to a bicuspid valve (6) and only contains 2 papillary muscles (anterolateral and posteromedial) (7), neither of which is positioned on the septum; it is slightly trabeculated (8) and its outflow tract includes a fibrous part in the continuity between the aortic valve and anterior leaflet of the mitral valve (9). The height difference between the septal insertions of the tricuspid and mitral valves (10) helps differentiate the RV (low insertion) from the LV (high insertion) (0-20° 4-chamber view). This indicator is lost if AV canal defects are present as both valves are inserted at the same level in patients with this pathology.
A right ventricle is distinguished by:
- The presence of a tricuspid valve inserted lower than the mitral valve (except if AV canal defects are present);
- Three papillary muscles, one of which is inserted into the interventricular septum;
- Thick trabeculation and the presence of a moderator band (a muscular trabecula that crosses the chamber);
- A conical and entirely muscular outflow tract (infundibulum) – there is no continuity between the tricuspid and pulmonary valves, which are normally separated from each other by the left outflow tract.
A left ventricle can be identified by:
- The presence of a bicuspid (mitral) valve inserted above the tricuspid valve (except if AV canal defects are present);
- Two (or just one) papillary muscle(s), neither of which has an insertion on the interventricular septum;
- Finer trabeculation;
- A partially fibrous outflow tract with continuity between the mitral valve and aortic valve.
Ventriculoarterial junction
The junction is concordant or discordant depending on whether or not the ventricle is connected to the appropriate vessel. Transposition of the great arteries (TGA) is the most common cause of ventriculoarterial (VA) discordance: the RV is connected to the aorta and the LV to the PA. A double-outlet ventricle is characterised by a layout in which over 50% of a ventriculoarterial valve is connected to the same ventricle, whether this be right, left or single (Figure 14.32). While the VA valves may exhibit overriding, straddling is impossible as they have no chordae.
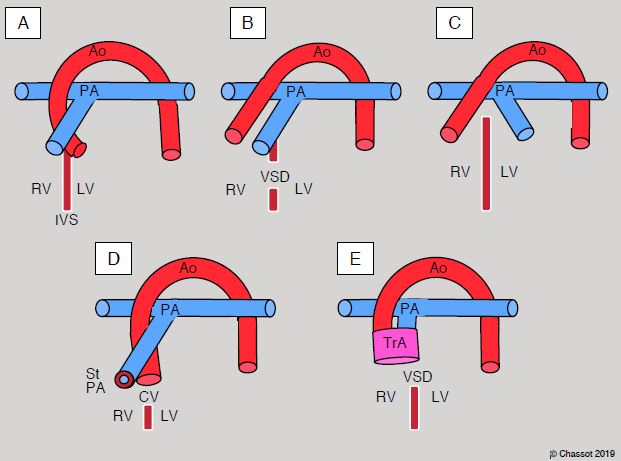
Figure 14.32: Conditions of the ventriculoarterial junction. A: normal situation – the aorta (Ao) and pulmonary artery (PA) cross at 45°, whereby the PA is anterior. The PA can be identified by its division into two branches 2-4 cm after its origin. The ascending aorta is a long tube that has no branches before the vessels of the aortic arch. Since numerous coronary artery anomalies are possible, the positioning of the coronary arteries cannot be used as a criterion for identifying the aorta. IVS: interventricular septum. B: double-outlet right ventricle (DORV) – due to the septum shifting, with or without VSD, both vessels originate from the RV. C:Transposition of the great arteries (TGA) – the aorta emerges from the RV and the PA from the LV. On an echocardiogram, the two vessels are parallel in the long-axis view at 120° and the two valves are on the same plane in short-axis view (0-40°). Generally, the great arteries are parallel in the sagittal plane in cases of D-TGA (anterior aorta, posterior PA) and side-by-side in the frontal plane in cases of L-TGA. D: Tetralogy of Fallot – the aortic root is widened and displaced to the right. It straddles the VSD. The PA is stenosed at its origin. E: truncus arteriosus – the aorta and PA emerge from the same vessel in different combinations. This vessel is open to both ventricles [4].
Arterial segment
Two vessels emerge from the heart – the aorta, which is posterior, and the pulmonary artery (PA), which is anterior. In normal circumstances, they cross each other at their origin. They can be differentiated by the fact that the aorta's first branches only emerge distally in the aortic arch, while the PA splits into two branches after 2-4 cm already. The coronary arteries are not a distinguishing feature as they may emerge from the aorta or the PA (ALCAPA: anomalous left coronary artery from pulmonary artery). If their embryonic septation has not occurred, the two vessels merge to form a single vessel (truncus arteriosus) [4].
Shunts and obstructions
Shunting involves communication between the systemic and pulmonary circulation and may occur in different locations.
Two vessels emerge from the heart – the aorta, which is posterior, and the pulmonary artery (PA), which is anterior. In normal circumstances, they cross each other at their origin. They can be differentiated by the fact that the aorta's first branches only emerge distally in the aortic arch, while the PA splits into two branches after 2-4 cm already. The coronary arteries are not a distinguishing feature as they may emerge from the aorta or the PA (ALCAPA: anomalous left coronary artery from pulmonary artery). If their embryonic septation has not occurred, the two vessels merge to form a single vessel (truncus arteriosus) [4].
Shunts and obstructions
Shunting involves communication between the systemic and pulmonary circulation and may occur in different locations.
- Venous return: pulmonary veins drained into a collector in continuity with the RA;
- Atria: ASD;
- Crux of the heart: AV canal defect;
- Ventricles: VSD;
- Arteries: patent ductus arteriosus, aorta-PA collaterals.
Obstructions mainly occur in the two ventricles’ outflow tracts and in the aorta. They may be dynamic (concentric muscular hypertrophy of the outflow tract) or static (subvalvular membrane, valvular stenosis, arterial stenosis).
- Right tract: dynamic stenosis of the RVOT, atresia or stenosis of the pulmonary valve, hypoplasia of the PA (typical of tetralogy of Fallot);
- Left tract: membrane in the LVOT, aortic stenosis (bicuspid aortic valve);
- Aorta: hypoplasia, coarctation.
Obstructive lesions cause excessive afterload in the affected ventricle, which undergoes concentric hypertrophy. If a shunt is present, these lesions modify the pressure ratio between the right and left circulation.
| Nomenclature |
|
In segmental analysis, the heart is divided into three segments (atrial, ventricular and arterial) and 2 junctions (atrioventricular and ventriculoarterial). The anatomical examination of congenital heart diseases is a 5-step process:
- Defining the situs based on the RA - Defining the various chambers (number, position) - Examining the AV and ventriculoarterial junctions (concordance or discordance) - Checking for shunts - Checking for obstructive lesions |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- ANDERSON R, HO SH: Echocardiographic diagnosis and description of congenital heart disease: Anatomic principles and philosophy. In ST JOHN SUTTON MG (ed): Textbook of echocardiography and Doppler in adults and children. Cambridge (MA): Blackwell Science, 1996, 711-743
- BETTEX D, CHASSOT PG. Transesophageal echocardiography in congenital heart disease. In: BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1186-1212
- DUPUIS C, KACHANER J, FREEDOM R, PAYOT M, DAVIGNON A. Cardiologie pédiatrique, 2ème édition. Paris, Flammarion, 1991, pp 137-42
- KLOESEL B, DINARDO JA, BODY SC. Cardiac embryology and molecular mechanisms of congenital heart disease – A primer for anesthesiologists. Anesth Analg 2016; 123:551-69
- PICCOLI GP. Morphology and classification of complete atrioventricular defects. Brit Heart J 1979; 42:633-9
- SHINEBOURNE EA, MACARTNEY FJ, ANDERSON RH. Sequential chamber localization. Logical approach to diagnosis in congenital heart disesase. Brit Heart J 1976; 38:327-40
- VAN PRAAGH R. Terminology of congenital heart disease. Glossary and commentary. Circulation 1977; 56:139-43
