Video: Leakage of cardioplegia solution into the LV in cases of even minor aortic insufficiency; this regurgitation can lead to dangerous dilation of the ventricle as soon as it stops ejecting.
At the end of ECC, the LV must have sufficient preload reserve to function adequately, as its size makes it inefficient at low filling volumes (Figure 11.142). The LV remains dilated even when filling pressures are immediately reduced, so some hypervolaemia and high preload must be maintained. However, a large-diameter ventricle is a poor pressure pump because its wall stress is higher than that of a small ventricle to eject the same stroke volume (Laplace's law). Beta-catecholamine support is necessary, as is arterial vasodilatation in hypertension. Restoring normal diastolic pressure will ensure good coronary perfusion.
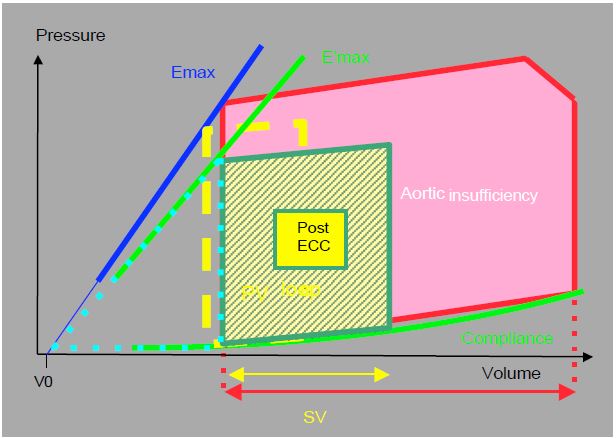
Figure 11.142: P/V loop of a patient with aortic insufficiency before (AI, in red) and after (green and yellow) aortic valve replacement. The competence of the prosthesis reduces diastolic filling and shifts the P/V loop to the left; systolic pressure and volume fall [from ref 8].
Aortic valve and root surgery
The many aetiologies of AI require a range of different interventions, and its association with dilatation of the aortic root sometimes requires specialised surgery (for details of prostheses, see Heart valve prostheses; for details of plasty, see Other heart valve repairs).
- Cusp repair: preferable to prosthesis if possible, as anticoagulation is not required and the rate of thromboembolism or endocarditis is very low. The operative mortality rate is around 1% and the 10-year success rate is 87%; the immediate failure rate is 14% [1]. The type of repair varies according to the valve pathology (Figure 11.60) [2,7,10].
- AI type Ia (dilatation of the sino-tubular junction): remodelling of the sino-tubular junction with a tubular aortic prosthesis, resuspension of the commissures, possibly subcommissural annuloplasty.
- AI type Ib (dilatation of the sinuses of Valsalva): remodelling of the aortic root by proximal anastomosis of a Dacron prosthesis (see Tirone David and Yacoub operations).
- AI type Ic (dilatation of the aortic ring): internal or external annuloplasty, reimplantation of the valve in a Dacron tube.
- AI type Id (perforation): closure with a pericardial patch.
- AI type II (prolapse): plication of the prolapsing zone around the Arantius node, triangular resection, annuloplasty, resuspension.
- Bicuspid valve: tricuspidisation, commissuroplasty.
- Prosthetic valves (see prosthetic valves).
- Mechanical prosthesis: excellent long-term results, but lifelong anticoagulation (INR 3.0-3.5 for the first 3 months, then 2.0-3.0 with aspirin); preferably indicated under the age of 65.
Video: Transgastric 120° long-axis view of a St-Jude mechanical aortic prosthesis; both wings open and close normally; major concentric hypertrophy of the LV.
- Fitted bioprosthesis: after 3 months of anticoagulation (INR 2.0-3.0), only aspirin is required; preferably indicated > 65 years of age or if there is a contraindication to anticoagulation; 20-30% of bioprostheses are dysfunctional after > 10 years.
Video: 30° short-axis view of a mounted bioprosthesis; presence of the 3 posts supporting the cusps at 3 o'clock, 7 o'clock and 11 o'clock.
- Stentless bioprosthesis: As stented bioprostheses always have a certain degree of stenosis, especially in small sizes, stentless valves are a good alternative for patients with a small aortic annulus; however, they are technically more difficult to implant.
Video: 40° short-axis view of a stentless bioprosthesis, characterised by the absence of posts and ring; free and normal movements of the 3 cusps.
Video: 120° long-axis view of the colour flow in the case of the previous video; absence of residual insufficiency and ejection stenosis. The sleeve of periaortic haematoma protruding into the LA is normal for the first few days postoperatively.
- Bioprostheses without fixation (sutureless): implantation of a prosthesis in the ECC by self-expansion or balloon inflation as with percutaneous prostheses, but with excision of the native valve.
A range of surgical techniques can be used to adapt the operation to the type of aortic root pathology.
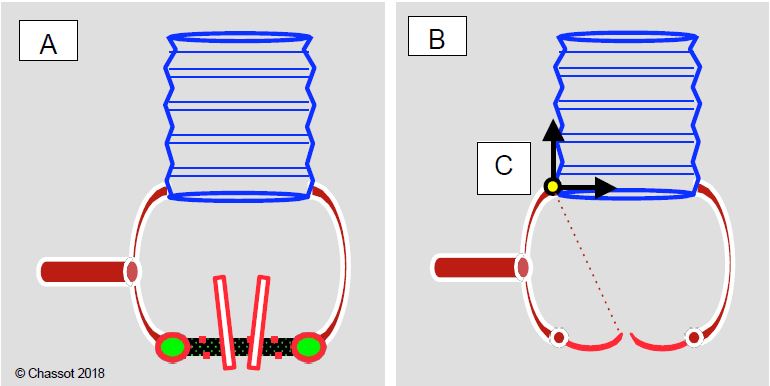
Figure 11.143: Schematic of ascending aortic procedures when the valve annulus and sinuses of Valsalva are normal. A: If there is pathology of the aorta and aortic valve, the tubular part of the aorta can be replaced separately with a Dacron™ tube and the valve with a prosthesis (mechanical or biological); the coronary arteries remain in place. B: If the aortic valve does not leak or has only mild to moderate insufficiency, only the tubular part of the aorta is replaced. C: By adjusting the diameter of the tubular prosthesis so that the dilated sinotubular junction has the same diameter as the aortic annulus, the cusps can be resuspended and brought to normal coaptation without changing the valve.
- Reimplantation of the native aortic valve in a Dacron tube fixed to the annulus at the junction of the LVOT and anastomosed distally to the ascending aorta (AI type Ic); the coronaries are reimplanted in the prosthesis; no anticoagulation [4].
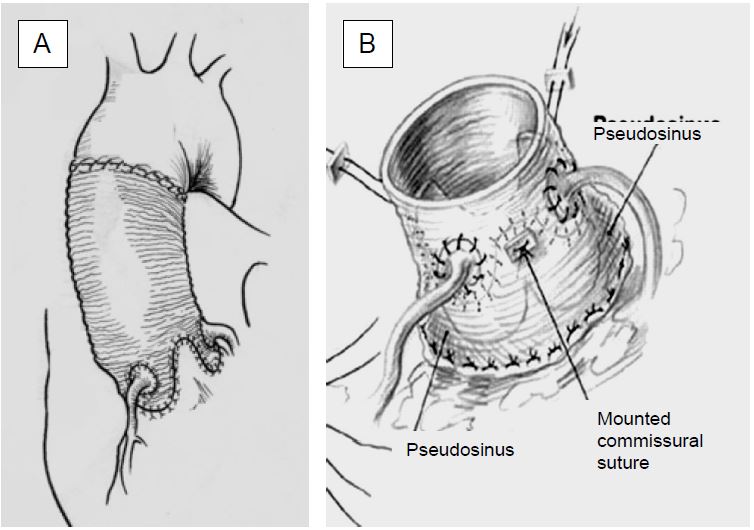
- Tirone David and Yacoub operation (Figure 11.144): resuspension of the aortic valve by proximal implantation of the tubular prosthesis replacing the ascending aorta and encompassing the sinuses of Valsalva (AI type Ia and Ib); reimplantation of the coronary arteries is necessary, but preservation of the native valve eliminates anticoagulation [4].
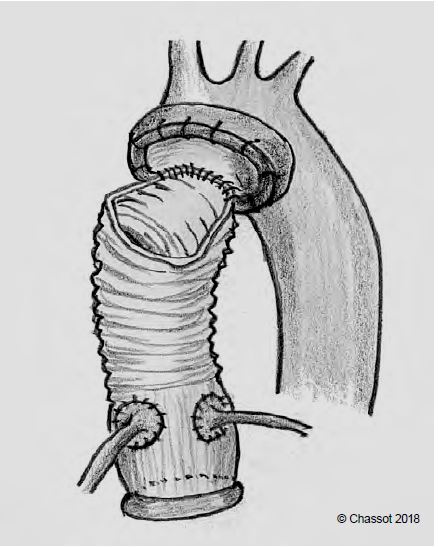
- Bentall operation (Figure 11.145): if the aortic annulus, the sinuses of Valsalva and the junction between the valve and the aorta are dilated and the tissue is pathological, the valve and the ascending aorta are replaced en bloc, with reimplantation of the coronary arteries in the prosthesis; this is the only guarantee against recurrence of dilatation of the aortic tissue left in place by the two previous operations. Anticoagulation is required depending on the type of prosthesis used (mechanical or biological).
Figure 11.144: Aortic valve prosthesis for aortic root dilatation. A: Resuspension of the aortic cusps by three-leaf clover incision of the ascending aortic prosthesis and coronary reimplantation (Yacoub operation); the diameter of the aortic root must be < 3 cm. Persistent aortic root tissue may lead to recurrent dilatation and recurrence of AI. B: Resuspension and incorporation of the aortic valve and annulus into the tubular prosthesis, coronary reimplantation (Tyrone David operation).
Figure 11.145: In the Bentall procedure, the prosthesis consists of a single mechanical or biological prosthetic valve and a tube. The operation consists of replacement of the aortic valve and ascending aorta and reimplantation of the right coronary artery and common trunk into the prosthesis. In the case of aneurysmal lesions of the aortic root, the tubular part of the prosthesis is short and the distal anastomosis is made in the middle of the ascending aorta. In this case, the distal anastomosis is performed on the proximal part of the saphenofemoral junction, with an open clamp in circulatory arrest to allow cushioning of the saphenofemoral junction orifice and suturing of the aortic wall and membrane in case of dissection.
- LV-Descending Aorta Valved Conduit: In the event of multiple reoperations, it is possible to short-circuit the aortic root using a Dacron tube fitted with a valve prosthesis; the system is implanted in the apex of the LV and anastomosed termino-laterally to the descending aorta; the operation is performed via a left thoracotomy [3].
- Homograft: an aortic valve with a sleeve of ascending aorta taken from a human cadaver and preserved in liquid nitrogen (-196°C). Although it has an accelerated wear rate, it has an excellent haemodynamic profile, causes few emboli and is resistant to infection; it is particularly indicated for valve replacement in endocarditis [5]. It can be used as a subcoronary valve, implanted in the moderately dilated sinuses of Valsalva (mini-root replacement), or as an aortic root replacement with reimplantation of the coronary arteries (full-root replacement).
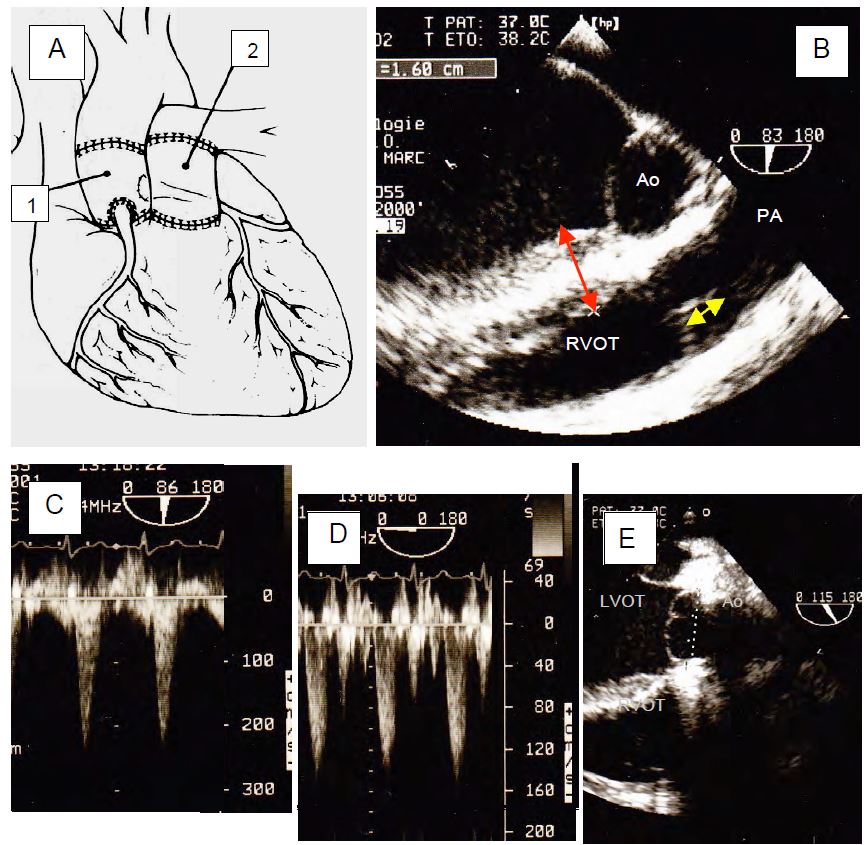
- Ross procedure (Figure 11.146): transposition of the pulmonary valve to the aortic position (with reimplantation of the coronary arteries) and placement of a homograft or heterograft in the pulmonary position. This complex and difficult technique avoids the need for a mechanical prosthesis and therefore anticoagulation; in children it allows the valve to grow to adult size. Operative mortality is 3% and long-term mortality < 1%/year; the rate of deterioration of the aortic autograft is 1%/year, but that of the right homograft/heterograft is higher (2-3%/year) [9]. The advantage of this strategy is that the valve is native to the aortic position.
Video: 30° short-axis view of the aortic neo-valve after Ross surgery; movement and occlusion are satisfactory.
If the homograft/heterograft degenerates, the pulmonary artery can accommodate a failure, even a major one; if the homograft suffers a stenosis, it can be dilated by catheterisation without risk of systemic embolism. The same wear lesions in the aortic position would require the use of a prosthesis. Contraindications to surgery include dilatation of the aortic root, bicuspid pulmonary valve, and a discrepancy of > 3 mm between the diameter of the aortic annulus and that of the pulmonary annulus. After bypass, the aortic valve must be competent (tolerated AI: degree ≤ minor), coapt with the annulus (no prolapse) over a height of > 4 mm, and have a maximum gradient < 20 mmHg; if these conditions are not met, a return to ECC is required. Antihypertensive medication reduces stress on the aortic neovalve and anti-inflammatory treatment extends pulmonary homograft survival [9].
Figure 11.146: Ross operation. After excision of the aortic valve, the patient's pulmonary valve is removed with a right pericardial sleeve, transplanted into the aortic position (autograft), and the coronary arteries are reimplanted. The pulmonary valve is replaced with a homograft or heterograft. A: Illustration of the procedure. 1: Autograft and aortic neo-valve. 2: Pulmonary homograft. B: Satisfactory coaptation height of the pulmonary valve (yellow arrow). Harvesting the vestibule involves longitudinal dissection of the interventricular septum; the pre-ECC examination must measure its thickness below the pulmonary valve (red arrow, 1.6 cm) to indicate to the surgeon the depth to which he can incise the septum without risk of creating a communication with the right vestibule (VSD). Cutting the first septal artery at this level may result in septal necrosis and a postoperative IVC. C: Neoaortic flow after stress ( ΔPmax 18 mmHg). D: Neopulmonary flow after bypass through the heterograft ( ΔPmax 8 mmHg). E: Long-axis view of the pulmonary neovalve; the result is unsatisfactory because the cusps prolapse into the outflow tract below the plane of the annulus (dotted line) and the height of coaptation is less than 1 mm. Although AI is not a concern in the immediate post-operative period, revision is inevitable in the longer term.
In all of these procedures, intraoperative TEE is of paramount importance to assess the condition of the valve and aortic root and to decide which type of intervention is most appropriate in each case. A number of key questions must be answered.
- Is AI secondary to cusp or root disease?
- Is it possible to keep the valve and just resuspend the leaflets?
- If the leaflets are affected or in the case of bicuspid valve, can surgery be performed?
- Can the sinuses of Valsalva and the sinotubular junction be preserved?
- Does the dilatation of the aortic root and the pathology in question require the valve and the ascending aorta to be replaced en bloc (Bentall)?
Although it gives very poor results in cases of calcified, degenerative or rheumatic lesions, aortic valve repair is an excellent technique for AI associated with bicuspid aortic valve, prolapse or dilatation of the aortic root. The technique is reserved for specialised centres as it requires considerable experience in this type of surgery, but it allows the native valve to be preserved, avoids the need for anticoagulation and minimises the risks of infection and thromboembolism. The re-operation rate at 10 years is 13-15% [6].
| Surgery in case of aortic insufficiency |
| Major risk of acute LV dilatation during bradycardia, ventricular fibrillation and cardioplegia at the start of ECC; careful monitoring of LV volume on TEE. Alpha vasopressors should not be used prior to aortic clamping in the presence of hypotension (elevated AI).
Surgery depending on the aetiology of the AI and the dilatation of the aortic root:
- Cusp repair or replacement with a prosthesis (AVR)
- Resuspension of the valve by the ascending aortic prosthesis
- Resuspension of the cusps by the aortic sleeve of the prosthesis, coronary reimplantation
- Plastie or AVR + ascending aortic replacement
- En-bloc replacement of valve, aortic root and ascending aorta (Bentall)
- Autotransplantation of the pulmonary valve into the aortic position (Ross procedure)
Post-ECC: The large size of the LV means that a high preload and inotropic support (dobutamine) must be maintained; avoid arterial hypertension (excessive afterload).
|
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- AICHER D, FRIES R, RODIONYCHEVA S, et al. Aortic valve repair leads to a low incidence of valve-related comnplications. Eur J Cardio-Thorac Surg 2010; 37:127-32
- BASMADJIAN L, BASMADJIAN AJ, STEVENS LM, et al. Early results of extra-aortic annuloplasty ring implantation on aortic annular dimensions. J Thorac Cardiovasc Surg 2016; 151-1280-5
- CRESTANELLO JA, ZEHR KJ, DALY RC, et al. Is there a role for the left ventricle apical – aortic conduit for acquired aortic stenosis ? J Heart Valve Dis 2004; 13:57-62
- DAVID TE. Aortic valve sparing in different aortic valve and aortic root conditions. J Am Coll Cardiol 2016; 68:654-64
- KARCHMER AW LONGWORTH DL. Infections of intracardiac devices. Infect Dis Clin North Am 2002; 16:477-89
- MINAKATA K, SCHAFF HV, ZEHR KJ, et al. Is repair of aortic valve regurgitation a safe alternative to valve replacement ? J Thorac Cardiovasc Surg 2004; 127:645-53
- PRODROMO J, D'ANCONNA G, AMADUCCI A, et al. Aortic valve repair fort aortic insufficiency: a review. J Cardiothorac Vasc Anesth 2012; 26:923-32
- ROSS J. After-load mismatch in aortic and mitral valve diases: Implications for surgical therapy. J Am Coll Cardiol 1985; 5:811-5
- TAKKENBERG JJM, KLIEVERIK LMA, SCHOOF PH, et al. The Ross procedure: a systematic review and meta-analysis. Circulation 2009; 119:222-8
- VAN DYCK MJ, WATREMEZ C, BOODHWANI M, et al. Transesophageal echocardiography evaluation during aortic valve repair surgery. Anesth Analg 2010; 111:59-70