- Tachycardia reduces diastolic filling, which is very slow;
- Bradycardia reduces flow because the systolic volume is fixed and low.
Pulmonary hypertension (PHT) is common in mitral stenosis because mean PLA remains elevated throughout the cardiac cycle, whereas in MI it is elevated only during systole. This chronic increase in PLA affects the pulmonary veins and induces pulmonary venous hypertension by stasis (postcapillary hypertension). The accumulation of interstitial fluid impairs gas exchange, leading to dyspnoea. Work of breathing is increased. This is followed by a reactive increase in PAR, which becomes fixed in the long term (precapillary hypertension). This is mainly responsible for the increase in LV afterload, as it is out of proportion to the left atrial pressure [3]. It is an adaptive phenomenon that restricts flow to the left heart, reduces blood accumulation in the LA and limits the risk of pulmonary oedema [9,11]. This leads to a cascade of right heart dysfunction: RV hypertrophy and dilatation, tricuspid regurgitation, RA dilatation and systemic venous stasis (see Figure 11.86). When resting systolic PAP exceeds 50 mmHg (mean PAP ≥ 35 mmHg), the functional limits of the LV no longer allow exercise, as PAP increases disproportionately. This combination of pre- and post-capillary PHT triples mortality [7]. The prevalence of PHT is 75% in mitral stenosis but approximately 30% in mitral regurgitation [5]. In general, mitral stenosis is characterised by LV dysfunction but preserved LV function.
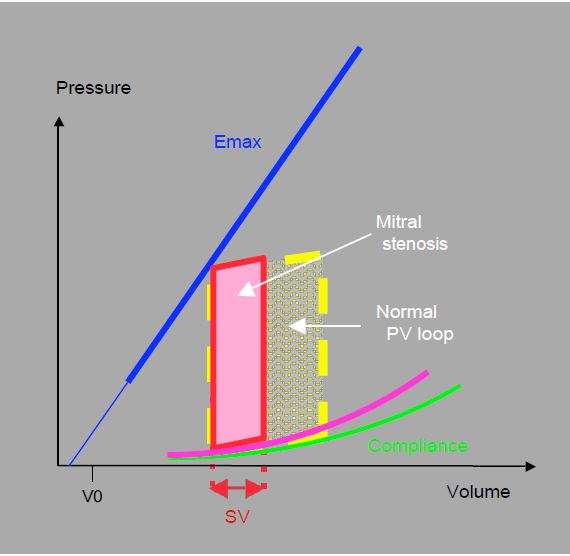
Although the left ventricle retains its contractile function, it is unable to vary its output because its filling capacity is limited. It operates at a low and fixed diastolic volume and is unable to increase its rate: when it accelerates, diastolic filling becomes inadequate. It has no preload reserve to modify its position on the Starling curve and improve its systolic performance during exercise: the systolic volume also remains low and fixed. Nor can the LV compensate for arterial vasodilatation or hypovolemia by tachycardia. The P/V loop (Figure 11.90) illustrates the typical haemodynamic situation: normal intraventricular pressures, limited stroke volume (< 50 mL), normal ejection fraction (EF 0.5 - 0.6); ejection work is reduced but pressure work is normal. Stroke volume is limited by the flow through the stenosis and the duration of diastole. Systemic arterial pressure, which is the product of cardiac output and systemic arterial resistance (SAP ≈ CO · SAR), is essentially dependent on SAR, which must remain high.
Figure 11.90: Pressure-volume loop in mitral stenosis. The restriction to ventricular filling results in a very small ventricular volume at values of Emax and compliance that are still normal. However, this is not always the case: in rheumatic myocarditis, for example, the LV is dysfunctional, the slope of Emax is reduced and the compliance curve is straightened. Stroke volume (SV) and ejection work are reduced, but pressure work is normal.
| Hemodynamic features of mitral stenosis |
| LV filling failure: low, fixed stroke volume Slow diastolic LV filling: intolerance to tachycardia Intolerance to hypovolemia Massive increase in PLA on exercise (tachycardia) or hypervolaemia Dilatation of LA: risk of AF and thrombus Preserved LV systolic function Stasis and pulmonary hypertension: venous (postcapillary) and arterial (precapillary) PHT Pressure overload of RV: dilatation, HRV, dysfunction, tricuspid insufficiency |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BLAIS C, PIBAROT P, DUMESNIL JG, et al. Comparison of valve resistance with effective orifice area regarding flow dependence. Am J Cardiol 2001; 88:45-52
- CHOI BW, BACHARACH SL, BARBOUR DI, et al. Left ventricular systolic dysfunction, diastolic filling characteristics and exercise cardiac reserve in mitral stenosis. Am J Cardiol 1995; 75:526-33.
- GERGES C, GERGES M, LANG MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in "out-of-proportion" pulmonary hypertension. Chest 2013; 143:758-66
- GORLIN R, GORLIN SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and the central circulatory shunts. I. Am Heart J 1951; 41:1-29
- HADDAD F, KUDELKO K, MERCIER O, et al. Pulmonary hypertension associated with left heart disease: Characteristics, emerging concepts, and treatment strategies. Progr Cardiovasc Dis 2011; 54:154-67
- HAKKI A, ABKULMASSIH IS. A simplified formula for the calculation of stenotic cardiac valves. Circulation 1981; 63:1050-5
- LIM GB. Prognostic relevance of pulmonary hypertension in valvular disease. Nat Rev Cardiol 2015; 12:194
- MEISNER JS, KEREN G, PAJARO OE, et al. Atrial contribution to ventricular filling in mitral stenosis. Circulation 1991; 84:1469-75
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- OTTO CM. Mitral stenosis. In: Otto CM, ed. Valvular heart disease 2nd edition. Philadelphia, WB Saunders, 2004; 252-5
- VACHIÉRY JL, ADIR Y, BARBERÀ JA, et al. Pulmonary hypertension due to left heart disease. J Am Coll Cardiol 2013; 62:D100-8