If atrioventricular discordance is combined with ventriculoarterial discordance, survival is possible since the systemic and pulmonary circulations are back in sequence. This is the case in congenitally corrected transposition of the great arteries or L-TGA. In cases of this anomaly, the PA and aorta are transposed as in TGA. However, the ventricles are also inverted. Consequently, the blood follows a physiological sequence through the crossed chambers: RA → LV → PA → lungs → LA → RV → Ao (Video and Figures 15.57 and 15.58).
Video: Congenitally corrected transposition of the great arteries; the TGA, not visible on this view, is doubled by a transposition of the ventricles: the LV is on the right side and the RV on the left; RV is recognisable by the presence of a septal papillary muscle, and by the septal insertion of the tricuspid valve below the insertion of the mitral valve.
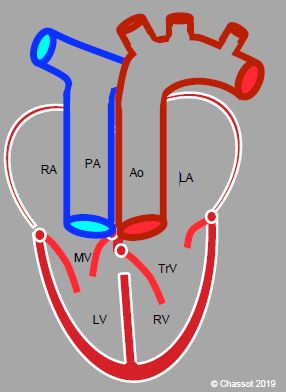
Figure 15.57: Congenitally corrected transposition of the great arteries (L-TGA). An atrioventricular discordance is combined with the ventriculoarterial discordance (TGA). The RA is connected to a mitral valve (MV) and an anatomically left ventricle (LV), and subsequently to the PA. Oxygenated blood is returned to the LA by the pulmonary veins. The LA drains through a tricuspid valve into an anatomically right ventricle, which is connected to the aorta. Although the patient is not cyanotic, his/her systemic ventricle is a right ventricle, which fails in 20-30 years’ time.
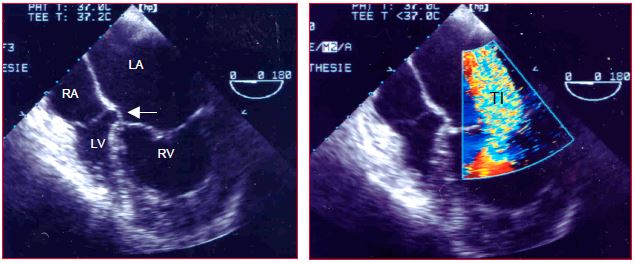
Figure 15.58: Congenitally corrected TGA. The IVC drains into the RA (situs solitus) connected to the LV, which is itself connected to the PA. The LA is connected to the RV, which is identified by the low insertion of the tricuspid valve (arrow). The RV is in a sub-aortic position. The RV is hypertrophied, but its high afterload (systemic pressure) has caused severe tricuspid insufficiency (TI).
The coronary artery system is inverted with the RCA on the left and the left main trunk on the right. Most patients present with a perimembranous VSD and anomalies of the atrioventricular valves, mainly of an Ebstein type [1,11].
Such patients are not cyanotic. However, the anatomically right ventricle is in a sub-aortic position. When functioning in this way as a systemic ventricle, the RV adapts to high afterload and increasingly resembles the LV. Circular contraction exceeds longitudinal contraction, contrary to a normal RV [8]. However, after functioning for around twenty years under these conditions, it dilates, develops severe tricuspid insufficiency, and fails between the ages of 25 and 40 years. The interventricular septum thus bulges into the LV and AV blocks are common [4]. The condition is often diagnosed at this stage. Mortality is 16% at 40 years [5]. In adults, surgical treatment is focused on tricuspid insufficiency (repair or replacement). This requires surgery before RV function deteriorates, and on closure of the VSD [2,3,9,10]. Anatomical correction is possible in most cases if diagnosis is established sufficiently early. However, the LV must first be prepared to take on systemic pressure by banding the pulmonary artery to increase its afterload and correct the position of the interventricular septum. The aorta and PA are subsequently retransposed (with a conduit where appropriate) [6]. Long-term survival (10-20 years) is 75-85% [7]. The only permanent treatment is transplantation, since the results of chronic medical treatment of right-sided failure are very disappointing in congenital heart disease patients.
Since the RV ensures systemic perfusion, it requires inotropic support and optimal coronary perfusion. The main challenges for anaesthesia are systolic failure of the systemic RV, a risk of RV ischaemia, and arrhythmias. SVR must be kept sufficiently but not excessively high to ensure coronary perfusion and increase afterload of the already failing RV. PiCCO™ is very useful for haemodynamic monitoring. TEE is essential for assessing ventricular function and blood volume.
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
Video: Congenitally corrected transposition of the great arteries; the TGA, not visible on this view, is doubled by a transposition of the ventricles: the LV is on the right side and the RV on the left; RV is recognisable by the presence of a septal papillary muscle, and by the septal insertion of the tricuspid valve below the insertion of the mitral valve.

Figure 15.57: Congenitally corrected transposition of the great arteries (L-TGA). An atrioventricular discordance is combined with the ventriculoarterial discordance (TGA). The RA is connected to a mitral valve (MV) and an anatomically left ventricle (LV), and subsequently to the PA. Oxygenated blood is returned to the LA by the pulmonary veins. The LA drains through a tricuspid valve into an anatomically right ventricle, which is connected to the aorta. Although the patient is not cyanotic, his/her systemic ventricle is a right ventricle, which fails in 20-30 years’ time.

Figure 15.58: Congenitally corrected TGA. The IVC drains into the RA (situs solitus) connected to the LV, which is itself connected to the PA. The LA is connected to the RV, which is identified by the low insertion of the tricuspid valve (arrow). The RV is in a sub-aortic position. The RV is hypertrophied, but its high afterload (systemic pressure) has caused severe tricuspid insufficiency (TI).
The coronary artery system is inverted with the RCA on the left and the left main trunk on the right. Most patients present with a perimembranous VSD and anomalies of the atrioventricular valves, mainly of an Ebstein type [1,11].
Such patients are not cyanotic. However, the anatomically right ventricle is in a sub-aortic position. When functioning in this way as a systemic ventricle, the RV adapts to high afterload and increasingly resembles the LV. Circular contraction exceeds longitudinal contraction, contrary to a normal RV [8]. However, after functioning for around twenty years under these conditions, it dilates, develops severe tricuspid insufficiency, and fails between the ages of 25 and 40 years. The interventricular septum thus bulges into the LV and AV blocks are common [4]. The condition is often diagnosed at this stage. Mortality is 16% at 40 years [5]. In adults, surgical treatment is focused on tricuspid insufficiency (repair or replacement). This requires surgery before RV function deteriorates, and on closure of the VSD [2,3,9,10]. Anatomical correction is possible in most cases if diagnosis is established sufficiently early. However, the LV must first be prepared to take on systemic pressure by banding the pulmonary artery to increase its afterload and correct the position of the interventricular septum. The aorta and PA are subsequently retransposed (with a conduit where appropriate) [6]. Long-term survival (10-20 years) is 75-85% [7]. The only permanent treatment is transplantation, since the results of chronic medical treatment of right-sided failure are very disappointing in congenital heart disease patients.
Since the RV ensures systemic perfusion, it requires inotropic support and optimal coronary perfusion. The main challenges for anaesthesia are systolic failure of the systemic RV, a risk of RV ischaemia, and arrhythmias. SVR must be kept sufficiently but not excessively high to ensure coronary perfusion and increase afterload of the already failing RV. PiCCO™ is very useful for haemodynamic monitoring. TEE is essential for assessing ventricular function and blood volume.
| Congenitally corrected transposition of the great arteries |
| An inversion of the ventricles is combined with the ventriculoarterial discordance of TGA. The blood follows a physiological sequence through the crossed chambers: RA → LV → PA → lungs → LA → RV → Ao. The RV is the systemic ventricle, limiting survival due to progressive right-sided failure and tricuspid insufficiency. |
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
- ANDERSON R, HO SH: Echocardiographic diagnosis and description of congenital heart disease: Anatomic principles and philosophy. In ST JOHN SUTTON MG (ed): Textbook of echocardiography and Doppler in adults and children. Cambridge (MA): Blackwell Science, 1996, 711-743
- BAUMGARTNER H, BONHOEFFER P, DE GROOT NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915-57
- BHATT AB, FOSTER E, KUEHL K, et al. Congenital hesart disease in older adult. A Scientific Statement from the American Heart Association. Circulation 2015; 131:1884-931
- CONNELLY MS, ROBERTSON P, LIU P, et al. Congenitally corrected transposition of the great arteries in adults: Natural history. Circulation 1994; 90:I-51
- DOBSON R, DANTON M, NICOLA W, et al. The natural and un-natural history of the systemic right ventricle in adult survivors. J Thorac Cardiovasc Surg 2013; 145:1493-501
- FILIPPOV AA, DEL NIDO PJ, VASILYEV NV. Management of systemic right ventricular failure in patients with congenitally corrected transposition of the great arteries. Circulation 2016; 134:1293-302
- HIRAMATSU T, MATSUMURA G, KONUMA T, et al. Long-term prognosis of double-switch operation for congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg 2012; 42:1004-8
- PETTERSEN E, HELLE-VALLE T, EDVARDSEN T, et al. Contraction pattern of the systemic right ventricle. J Am Coll cardiol 2007; 49:2450-6
- SILVERSIDES CK, SALEHIAN O, OECHSLIN E, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Complex congenital cardiac lesions. Can J Cardiol 2010; 26:e98-e117
- STOUT KK, DANIELS CJ, VALENTE AM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease. J Am Coll Cardiol 2019; 73:e81-192
- WARNES CA, WILLIAMS RG, BASHORE TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008; 118:2395-451
