Cardiac surgery often comes along with coagulopathy, which is multifactorial in nature but largely related to extracorporeal circulation (ECC) [18,47].
- Preoperative anticoagulant or antiplatelet medication ;
- Preoperative coagulopathy ;
- Hemodilution ;
- Hypothermia, acidosis ;
- Full heparinization for ECC, residual heparinization after protamine;
- Heparin resistance ;
- Activation and consumption of factors on foreign surfaces ;
- Activation and consumption by pericardial/pleural extravasation and suctions;
- Platelet activation and consumption, thrombocytopenia and platelet dysfunction ;
- Hyperfibrinolysis ;
- Systemic inflammatory syndrome.
ECC
Independent of tissue damage, ECC directly triggers the formation of thrombin and fibrin. Five minutes after procedure starts, their level is already increased by 20 times, although these substances are normally only found in the wound and not in the systemic circulation [4]. Several phenomena are involved [18,41].
- The contact system. On contact with negatively charged foreign surfaces such as glass or plastics, factor XII (Hageman) cleaves to XIIa (activated) which converts prekallikrein to kallikrein, factor XI to XIa and kininogens to bradykinin; the latter increases 10-fold. Activation of FXIa leads to the active formation of thrombin via the intrinsic coagulation pathway. Factor XIIa also activates the complement pathway and promotes the conversion of plasminogen to plasmin, leading to fibrinolysis. However, the balance is in favour of excess thrombin and the procoagulant effect; this situation persists until 5th postoperative day [2].
- The extrinsic pathway. Tissue factor (TF) expression and factor VIIa concentration are abnormally high in ECC.
- The level of circulating plasmin increases 10-50 times during ECC; as the rates of fibrin formation and degradation are equivalent, this situation amounts to an increased consumption of fibrinogen without clot formation [5].
- They are stimulated by contact with foreign surfaces, by heparin and by excess circulating thrombin; they adhere to surfaces, form clusters and secrete vasoconstrictor thromboxane A2 . Their number and aggregability decrease by 30-50% during ECC [39]. Their function is reduced in hypothermia (< 30°C), but this dysfunction is reversible on rewarming; however, it does not show up on the result of aggregability tests that are performed on blood warmed to 37°C. Plasmin dissociates the GP Ib receptor, which partially activates the platelet but makes it less sensitive to agonists [11].
- The inflammatory reaction. Leukocytes are activated by foreign surfaces and secrete tissue factor (TF) which contributes to the development of the coagulation cascade and production of thrombin. Foreign surfaces also stimulate the alternative complement pathway (the classical pathway is already activated by F XIIa); C3a and C5a factors bind to circulating leukocytes and contribute to their activation.
- The levels of all factors are lowered by 20-30% by water dilution of the ECC. Colloids lower factor VIII and von Willebrand levels and inhibit platelet adhesion [14,39].
- By the end of the ECC, fibrinogen levels have decreased by 30-40%; 30% of antithrombin III is consumed, which tends to progressively increase the "resistance" to heparin. Factors II, VII, IX and X are decreased by almost 50% after ECC [16].
- Retransfused blood. If not processed by a CellSaver™ system, blood aspirated from the pericardium or mediastinum contains tissue factor, thrombin-antithrombin complexes, plasminogen activators and inflammatory triggers. It contributes massively to coagulation alterations.
- Hypothermia and acidosis. Coagulopathy sets in at 35°C, and the coagulation cascade is completely inactivated at 16°C. Acidosis worsens the situation and slows down the activity of pH-sensitive factors such as factor VIIa.
The expression of tissue factor (TF) and the extrinsic pathway are primarily related to surgical trauma, activation by suctions and the inflammatory response. Activated leukocytes infiltrate between endothelial cells and produce free radicals, superoxides and lysosomal enzymes, resulting in endothelial damage, increased capillary permeability, extracellular fluid accumulation and systemic inflammatory syndrome (see Chapter 7 Inflammatory syndrome). Damage to platelets and coagulation factors (protein denaturation) is directly related to the duration of ECC, the depth of hypothermia (≤ 25°), suctions, and contact with air (venous reservoir, aspirations).
Activation of coagulation can be reduced by various means, but apart from anticoagulation, their effectiveness is highly variable.
- Complete anticoagulation with non-fractionated heparin (UFH); thrombin activity is blocked when ACT is > 480 seconds, provided that sufficient anti-thrombin is present (see Heparins). The loading dose of heparin to achieve adequate anticoagulation is 300-400 IU/kg. ACT is checked 3-5 minutes later. Additional doses are titrated according to the patient's individual response to heparin.
- In case of heparin resistance, supplementation with anti-thrombin (AT III), as its level decreases by 40% in ECC due to haemodilution and consumption by heparin. The nadir of AT III concentration is reached at 3rd postoperative day [10]. Administration in the form of TA III concentrate (500-1000 IU for an adult) or fresh frozen plasma (see below) [48].
- In patients on anticoagulants or antiplatelets, the dose of heparin used during ECC or OPCAB remains the same as the usual routine (ACT sought: > 450 sec and > 250 sec respectively), as incomplete inhibition of thrombin may lead to secondary platelet activation [3].
- Antifibrinolytics; tranexamic acid and amino caproic acid bind to the lysine of plasminogen and block plasmin activation, hence fibrinolysis. Aprotinin is a non-specific protease inhibitor, which directly blocks plasmin (see Anti-fibrinolytics).
- Thromboplegia; ECC and heparin activate platelets, which release their granules (ADP, thrombexane), form aggregates and adhere to surfaces; 30-50% of them are no longer functional postoperatively and no longer react to ADP or collagen [39]. Their momentary blockage by a P-receptor antagonist2 Y12 (ADP receptor) such as cangrelor in perfusion (half-life: 9 minutes) protects them from stimulation and preserves their functionality for the postoperative period [27]. This promising therapy is still in trial phase.
- Changes related to ECC technology [18].
- Suction restriction; recovered blood is in contact with air and contains activators of coagulation (TF, thrombin), fibrinolysis (plasmin) and inflammation (interleukins, TNF, C3a, C5a). Suction is the main source of haemolysis, thrombocytopenia, coagulopathy and stimulation of the inflammatory syndrome [41]. Disruption of the coagulatory system is markedly reduced when aspirated blood is not recycled or is filtered through a CellSaver™ system, but this manoeuvre unfortunately removes platelets, proteins and coagulation factors [46].
- Restriction of circuit size; miniaturisation of circuits and removal of the cardiotomy reservoir minimises contact of blood with foreign surfaces and eliminates contact with air, thereby reducing the release of coagulation activators and inflammatory triggers.
- Biocompatibility of circuits; preheparinised circuits and circuits impregnated with particular polymers inhibit the complement cascade, platelet aggregability and leukocyte activation. However, the clinical effect is small and limited to a decrease in postoperative AF and ICU stay [31]. The reduction in transfusions is not consistent [37].
- Ultrafiltration; continuous filtration at the end of the ECC after weaning off (MUF, modified ultrafiltration) reduces haemodilution and removes many cytokines and inflammatory triggers.
- Beating heart surgery without ECC; absence of ECC does not eliminate coagulo-inflammatory activation, but reduces it; platelet dysfunction is reduced [44].
Anticoagulation and coagulopathy related to ECC should be antagonised and treated respectively to limit bleeding risks. The antagonist of heparin is protamine, administered at a ratio of 1 mg protamine to 1 mg heparin (see Heparine and protamine below). Excess protamine can inhibit the coagulation cascade and platelet activity. Protamine is injected as soon as venous canula is out . It has several side effects:
- Systemic vasodilation and arterial hypotension ;
- Pulmonary vasoconstriction ;
- Antigen-antibody reaction ;
- Sudden anaphylactoid reaction (anaphylactic shock).
| Coagulopathy of the ECC |
|
ECC coagulopathy is related to 3 phenomena: haemodilution, activation and consumption. By contrast, formation of thrombin and fibrin is rapidly triggered by ECC, independent of any tissue injury. Five systems are activated by contact with foreign surfaces:
- Activated F XII triggers the intrinsic pathway
- Tissue factor and factor VIIa are increased
- Platelets are stimulated
- Activation of fibrinolysis destroys the fibrin formed but consumes fibrinogen
- Activation of leukocytes and complement triggers an inflammatory response,
Massive and systemic
Several means are used to compensate for this stimulation:
- Complete anticoagulation (heparin 300-400 IU/kg)
- Antifibrinolytic
- Suction restriction, CellSaver™
- Circuit restriction, biocompatible circuits
- Ultrafiltration
- Beating heart surgery
|
Anticoagulation in ECC
Heparin is essential for the smooth running of operation with ECC. For this reason it is injected via a central venous line after checking for blood reflux, or administered directly into the RA by the surgeon; the injection is followed by a flush to ensure that the full dose is injected. Whether administered by the surgeon or the anaesthetist, its injection is clearly announced so that the perfusionist can perform a follow-up ACT 3-5 minutes later. The loading dose of heparin to achieve adequate anticoagulation for ECC is 300-400 U/kg [11]. Thereafter, additional doses should be titrated according to the individual patient's response. The minimum ACT needed to avoid thrombotic or haemorrhagic problems during a bypass is still discussed, but a value of ≥ 450 seconds is generally accepted as a reference for standard circuits, and a value of ≥ 250 seconds for assistanting devices with pre-heparinised circuits. If the ACT is < 400 seconds, ECC is not started without adding an additional dose of heparin (5,000 - 10,000 IU); a repeat check is performed 3 minutes later.
Heparin resistance
Sometimes ACT does not reach the desired values despite a high dose of heparin. This resistance to heparin is usually due to antithrombin (AT III) deficiency from several causes [12].
- Congenital AT III deficiency (incidence 1:3,000); AT III levels are lowered by 40-60%.
- Consumption of AT III by ongoing heparin therapy; the fall in antithrombin is 5-10% per day. Preoperative treatment with heparin for several days is the most frequent cause.
- Haemodilution; plasma AT levels may decrease by 30%.
- Decreased AT III production in liver failure, malnutrition or nephrotic syndrome.
- Increased AT III consumption in sepsis, DIVC, pulmonary embolism or mechanical circulatory support.
Management is based on three elements.
- Increase in heparin doses. However, there is a ceiling effect: anticoagulation no longer deepens when the heparin level is > 4 U/mL [29].
- Fresh frozen plasma. FFP contains approximately 1 U of AT III per mL. Two bags of FFP are rarely enough to compensate for AT III deficiency [10]. The administration of enough AT III to normalise AT III (1-2 L) raises the possibility of hypervolaemia. On the other hand, FFP carries all the infectious and allergic risks associated with blood cell transfusions.
- Antithrombin concentrate, in purified human form or recombinant form (synthesised in genetically modified goat's milk). The half-life of the product is 12 hours and 3.8 days respectively. The recommended dose is 500-1,000 IU for an adult [48], which is relatively modest, as up to 45 U/kg is required to maintain normal ATIII levels [10]. The cost of this treatment is CHF 1,200-2,500, but it is more effective than FFP.
The most logical approach is to measure ATIII and only give antithrombin concentrate when level is low; if it is normal, an increase in heparin dosage is usually sufficient [12].
| Anticoagulation in ECC |
|
Contact of the blood with foreign surfaces and air requires complete anticoagulation with non-fractionated heparin (UFH). Heparin is administered centrally prior to ECC at 300-400 IU/kg to achieve an ACT > 450 seconds. If the ACT is < 400 seconds, an additional dose of 10,000 U is added and rechecked. If the ACT cannot be prolonged despite an adequate dose of UFH (heparin resistance), antithrombin III deficiency should be suspected and replaced with concentrate (500 - 1000 U/kg), possibly freshly frozen plasma.
Patients with heparin-induced thombocytopenia may be anticoagulated with other substances, unfortunately without antagonists and not reversible with protamine:
- Bivalirudin (Angiox® )
- Argatroban (Argatroban Injection® )
Protamine antagonises non-fractionated heparin at a ratio of 1 mg to 1 mg of heparin (100 IU).
|
Heparin and protamine
Protamine antagonises heparin in a 1:1 (mg) ratio. The dosage of protamine is overestimated if the calculation is based on the total amount of heparin administered, as it does not take into account elimination. It is therefore recommended to give only 80% of the dose or to titrate the residual heparin. Excessive protamine may increase bleeding, as it activates endothelial plasminogen secretion and binds to thrombin, thus blocking the conversion of fibrinogen to fibrin. Proper dosing is all the more important as protamine has important side effects: histamine release (systemic arterial hypotension), anaphylactic reaction, vasoplegic shock, pulmonary hypertension.
Hemodilution
The mixing of blood with priming fluid (800-1,500 mL of hydroelectrolyte solution) is responsible for a major haemodilution that suddenly lowers the haematocrit to around 25% and decreases the colloid osmotic pressure by 40% [21]. This is the main cause of pressure drop at beginning of ECC. The pressure then rises again because hypothermia causes a stimulation of peripheral arterial resistance (PAR) and because viscosity increases as the blood temperature drops. The fall in osmotic pressure exacerbates extracellular fluid leakage into interstitial space of lungs, heart, liver, kidneys, abdominal viscera and muscles.
Hemodilution is beneficial in several ways (see Chapter 7, Priming fluid).
- It improves microcirculation by lowering blood viscosity, which is crucial in hypothermia; viscosity remains stable when Ht in percent has the same value as temperature in degrees C°;
- It reduces need for allologous blood and complications associated with transfusion;
- It is well tolerated as O2 tissue is consumption reduced when cold.
In normothermic ECC, an Ht of 18% is just enough to meet oxygen requirements of a sedated and curarised patient [30]. When Hb is <70 g/L, cerebral blood flow increases by 45% and renal plasma flow rises in the cortical area, but coronary reserve decreases by 50% and splanchnic perfusion is borderline ischaemic [35,42].
Hemodilution is only beneficial within certain limits. A Ht below 22%, for example, is an independent predictor of postoperative morbidity and mortality [20]. Ht has a particular impact on brain function and renal function. Neurocognitive impairment becomes more important when the minimum Ht is 15-17% [8]; only Ht > 28% ensures normal postoperative neurological status [45]. In children, neurological score and psychomotor development are better at high Ht in ECC (28%) than at low Ht (21%) [23]. On the other hand, renal function worsens linearly with decreasing haemoglobin when haematocrit is < 30% [43]. A Ht of 25-28% during ECC is therefore a safe lower limit to ensure functional recovery of the organs.
Hemolysis
Contact with foreign surfaces of various kinds causes a series of haematological injuries of varying severity, which are generally directly proportional to the duration of ECC. In addition, pumps cause mechanical damage to blood cells, which depends on their degree of occlusivity and their speed of rotation. Finally, suctions in the operating field are responsible for some of the inflammatory and erythrocytic damage, the more serious the more powerful and prolonged the suctions are and the greater haemorrhage is. The resulting haemolysis is clearly obvious in urine bag, which becomes reddish-brown. In this case, it is necessary to maintain urine flow and to alkalinise urine with Na+ bicarbonate (50-100 mmoles i.v.) to slow down the crystallisation of free Hb in renal tubules [19].
Another cause of haemolysis is cold agglutinin. This is an autoimmune disease characterised by presence of antibodies that cause agglutination of erythrocytes below a certain temperature threshold. Cold agglutinins are IgM antibodies directed against Anti-Ig antigens present on the membrane of red blood cells. They cause agglutination of red blood cells at low temperatures. During ECC warming phase, these aggregates cause microvascular thrombi and are haemolyzed, releasing a large amount of free haemoglobin. This condition is an idiopathic disease, or the sequelae of an infectious or lymphoproliferative process. Its incidence is less than 1% of cardiac surgery patients. Patients display peripheral thrombosis and haemolysis (see Chapter 21 Coagulopathies).
Cold agglutinins are detected by both direct (presence of complement on the patient's RBCs) and indirect (presence of serum antibodies) Coombs tests. They occur in all individuals, but normally only react at 0-4°C. Their clinical significance lies in their serum level and the temperature value at which they are activated. The values considered safe for ECC are a concentration of less than 1:32 at 4°C, with no detectable agglutination at 28°C or above. The likelihood of intraoperative complications becomes significant for levels above 1:512 at 4°C, or below this value if the activation temperature is above 25°C [33].
In the operating room, a series of precautions are taken.
- Surgery in normothermia (ECC > 34°C) or beating heart;
- Warming up the operating room and infusions;
- Warm (> 34°C) crystalloid or blood cardioplegia;
- Warming of blood bags in case of transfusion ;
- In case of crisis with haemolysis :
- Warm to 37°C;
- Improve peripheral perfusion with a vasodilator (nitroprusside);
- Alkalinate the urine (50-100 mmoles Na bicarbonate+ );
- Methylprednisolone (500 mg): effectiveness discussed;
- Reduction of circulating levels by preoperative plasmapheresis if necessary.
Crises manifest through haemolysis and peripheral vascular occlusions of myocardium, liver and kidneys.
| Haemodilution and haemolysis in ECC |
|
Priming volume of ECC causes haemodilution (Ht 25-28%), which is necessary to slow increase in blood viscosity at low temperatures. In hypothermia, viscosity remains stable when the Ht in % is the same as the temperature in degrees C°. When Ht is < 25%, postoperative neurological status and renal function are impaired. A Ht of 25-28% is the safe lower limit to ensure normal organ function.
ECC causes haemolysis, usually sub-clinical. In hypothermia, this may become massive in presence of cold haemagglutinins (demonstrated by a Coombs test).
|
Cardiac surgery for HIT
When a patient has heparin-induced thrombocytopenia (see HIT), other anticoagulants are available, but are not antagonised and are not reversed by protamine. Elimination depends on their serum half-life (see Direct Thrombin Inhibitors, Table 8.1 and Table 8.2 ) and use of haemofiltration at the end of ECC [1,6,15,24,26].
- Bivalirudin (Angiox® ): reversible direct thrombin inhibitor. Onset of activity: 2-10 minutes; half-life: 25 min; renal elimination (extended half-life up to 200 minutes in renal failure). Easy to use with ECC, but with a risk of thrombosis in reservoir or oxygenator if machine flow is interrupted, as elimination of bivalirudin by plasma proteolysis continues in still blood. Therefore, a biocompatible circuit, arteriovenous shunts, a CellSaver™, intermittent flushing of the venous reservoir and storage of blood in citrated bags are required. Dosage: bolus 1 mg/kg + 50 mg in the ECC priming fluid + infusion 2.5 mg/kg/h. ACT ≥ 2.5 times baseline is targeted with additional boluses of 0.1-0.5 mg/kg; target circulating level on bypass: 10-15 mcg/mL [25,26]. The ecarin coagulation time (ECT) is a more specific test. Perfusion is stopped 10-15 minutes before end of bypass. Ultrafiltration is used after ECC to accelerate the elimination of bivalirudin. A return in pump is not possible after weaning.
- Argatroban (Argatroban Injection® ): a synthetic molecule derived from arginine that binds selectively and reversibly to thrombin. Half-life: 40-50 minutes; hepatic elimination, dosing independent of renal function. Dosage: bolus 0.1-0.2 mg/kg iv + 0.05 mg/kg in ECC priming fluid + infusion 5-10 mcg/kg/min (immediate and continuous) for ACT 350-400 sec; additional bolus if necessary: 2 mg. Return to normal coagulation 2-4 hours after stop of infusion. Argatroban is the drug of choice in cases of renal dysfunction, but can’t be eliminated through haemodialys.
- Danaparoid sodium (Orgaran® ): Predominantly inhibits factor Xa. Half-life of 7 hours for anti-IIa activity and 25 hours for anti-Xa activity; renal elimination. Dosage: bolus iv 1,500-2,000 U + 5,000-10,000 U added to ECC priming fluid; addition of 1,500 U after 2 hours. Very difficult to manage on bypass and very haemorrhagic in the postoperative period.
- Lepirudin (Refludan® ): recombinant form of hirudin, irreversible thrombin inhibitor. Serum half-life 10 min, elimination half-life 1-1.5 hours, but irreversible thrombin inhibition; renal elimination. Best monitored by ECT (ecarin clotting time of citrated whole blood). Dosage: bolus 0.25 mg/kg + 0.2 mg/kg in the ECC priming fluid + bolus 5 mg for ACT > 350 sec (imprecise); infusion 0.15 mg/kg/h. Lepirudin production ceased in 2012 for commercial reasons.
These substances have variable influence on coagulation tests [26]. ACT correlates well with circulating bivalirudin and argatroban levels. The aPTT is modified, but not linearly. PT is more affected by argatroban than by bivalirudin. More specific tests such as ecarin time or anti-IIa assay are unfortunately not available in all hospitals. Bivalirudin is the safest agent and is used in situations where there is concern about heparin-induced thrombocytopenia (HIT) or in situations where short term deep anticoagulation is required such as PCI [26].
- Bypass: bolus 1.0 mg/kg, infusion 2.5 mg/kg/hr;
- OPCAB: bolus 0.75 mg/kg, infusion 1.75 mg/kg/h;
- Intraoperative ECMO: bolus 0.2-0.5 mg/kg, infusion 0.2-0.5 mg/kg/h;
- ECMO in intensive care: start 0.03-0.05 mg/kg/min, continuous 0.03-0.1 mg/kg/min.
| ECC in heparin-induced thrombocytopenia (HIT) |
|
HIT (heparin-induced thrombocytopenia) is triggered by antibodies to the complex formed by heparin and platelet factor-4 (PF4), which results in activation of the coagulation chain. Characteristics :
- Heparin treatment for 5-10 days
- Thrombocytopenia (fall > 50%)
- Venous and arterial thrombosis
- High level of anti-heparin antibodies (attenuated beyond 3 months)
- Incidence: 1-5% with UFH, 0. 1-1% with LMWH, more frequent after surgery
- Mortality: 5-10%.
Treatment: discontinue heparin and replace with an alternative. Substances that can be used for anticoagulation in ECC:
- Bivalirudin (Angiox® ), easy to handle
- Argatroban (Argatroban Injection® ), preferable in cases of renal failure
- Danaparoid sodium (Orgaran® ), very slow, high bleeding risk
- Lepirudin (Refludan® ), withdrawn from market
|
Systemic inflammatory syndrome (SIRS) related to ECC
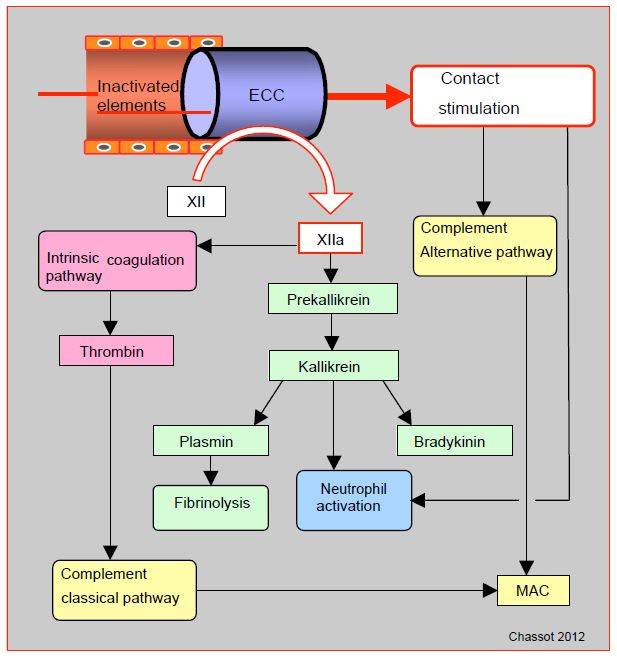
ECC is the most emblematic case of stimulation by the complement contact pathway (Figure 8.20) (see Chapter 07, Systemic inflammatory syndrome). In presence of negatively charged surfaces such as glass, metals or plastics, Factor XII cleaves to F XIIa (activated). This converts prekallikrein to kallikrein, kininogens to bradykinin and factor XI to F XIa; the latter process leads to formation of thrombin via the intrinsic coagulation pathway. F XIIa also promotes conversion of plasminogen to plasmin, causing fibrinolysis. Contact activates complement directly through the alternative pathway, and indirectly through factor XIIa (classical pathway). Other phenomena in ECC contribute to complement activation, such as the release of endotoxins from gastrointestinal tract and formation of heparin-protamine complexes. The cellular pathway is also stimulated by contact, either via factor XIIa and kallikrein or directly by neutrophil activation. However,extracorporeal circuit does not have an endothelium to limit these different reactions, which can therefore become excessive and spread throughout the body.
Figure 8.20: Activation via the contact pathway. In a vessel lined with intact endothelium, the various factors circulate in inactive form. But in the presence of negatively charged surfaces such as glass, metals or plastics (ECC), factor XII cleaves to (activated) F XIIa. This converts prekallikrein to kallikrein, kininogens to bradykinin and factor XI to F XIa; the latter process leads to the formation of thrombin via the intrinsic coagulation pathway. F XIIa also promotes the conversion of plasminogen to plasmin, causing fibrinolysis. Contact activates complement directly through the alternative pathway, and indirectly through F XIIa (classical pathway). MAC (membrane attack complex): a protein complex that attacks cell membranes, the ultimate result of the complement pathways.
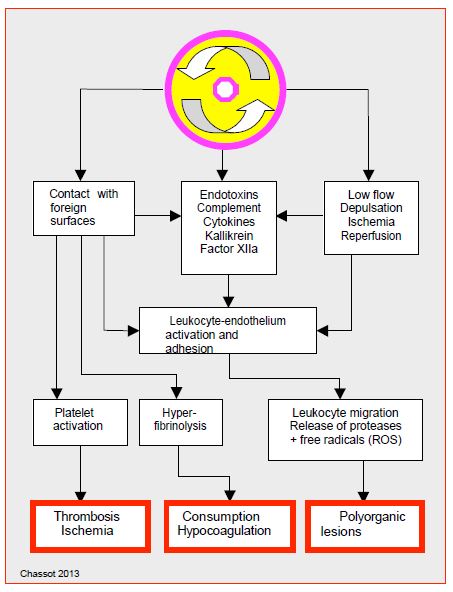
Duration of ECC, depth of hypothermia and degree of haemodilution have all been suggested as worsening factors, but appear to have a secondary role in genesis of SIRS [22]. Mechanical damage through pump, oxygenator and filters, contact of blood with foreign surfaces (circuits) and with air (aspirations, venous reservoir) are the main triggers. More than 50% of neutrophils are sequestered in lungs during rewarming; their degranulation contributes to lung cell damage. SIRS is triggered in the first minutes of the ECC and is terminated around 4th – 5th postoperative day, followed by a period of relative immunosuppression [38]. The peak of inflammatory markers occurs at around 5th hours post-ECC. Inflammatory stimulation during ECC thus leads to an unstable state featured by (Figure 8.21).
- Elevation of all inflammatory markers (TNF-alpha, interleukins, cytokines, CRP);
- Activation of complement and leukocytes;
- Thrombin production and stimulation of the coagulation cascade ;
- Production of plasmin and stimulation of fibrinolysis (elevation of D-dimer);
- Platelet activation and consumption ;
- Release of endotoxins and TNF-alpha ;
- Release of free radicals and oxidants;
- Release of bradykinin, histamine and anaphylatoxins: increased capillary permeability, systemic vasodilation, pulmonary vasoconstriction;
- Alterations in cardiac, pulmonary, renal and cerebral function; the incidence of atrial fibrillation is proportional to the elevation of inflammatory markers [13].
The question of how much of the genesis of SIRS is due to ECC versus cardiac surgery itself can be evaluated by observing beating heart surgery. The latter is associated with a reduction, but not an elimination, of postoperative levels of inflammatory response markers such as C3a, C5a, TNF-alpha or interleukin-6 and IL-8 [7,9,17]. However, the significance of these variations is uncertain, as IL-8 and C3a are related to direct tissue trauma and IL-10 has anti-inflammatory properties [32]. The effects of ECC depend largely on the balance between release of pro- and anti-inflammatory mediators [28]. Some populations show a dominant pro-inflammatory response, such as the elderly or those with left ventricular dysfunction [40]. They may particularly benefit from beating heart surgery. In low-risk groups, SIRS-related complication rates are identical between operations with and without ECC [34,36]. The bypass circuit is therefore a major triggering factor, but not the only one responsible for the inflammatory response (see Anti-inflammatory therapy) [44].
Figure 8.21: Schematic representation of the mechanisms involved in the genesis of the systemic inflammatory syndrome [from references 22,41].
| Inflammatory syndrome and ECC |
|
Direct contact of blood with bypass circuit and air induces a massive systemic inflammatory response (SIRS) triggered by the activation of Factor XII (FXa), complement and leukocytes. It is characterised by:
- Elevation of all inflammatory markers
- Activation of complement and leukocytes
- Thrombin production and stimulation of the coagulation cascade
- Plasmin production and stimulation of fibrinolysis
- Platelet activation and consumption
- Release of endotoxins, TNF-alpha and interleukins
- Release of free radicals and oxidants
- Release of bradykinin, histamine and anaphylatoxins
- Increase in capillary permeability, decrease in SAR, increase in PAR
- Alterations in heart, lung, kidney and brain function
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- ADAMS RLC, BIRD RJ. Review article: Coagulation cascade and therapeutic update: Relevance to nephrology. Part I: Overview of coagulation, thrombophilia and history of anticoagulants. Nephrol 2009; 14:462-70
- AVIDAN MS, LEVY JH, SCHOLZ J, et al. A phase III, double-blind, placebo-controlled, multicenter study on the efficacy of recombinant human antithrombin in heparin-resistant patients scheduled to undergo cardiac surgery requiring cardiopulmonary bypass. Anesthesiology 2005; 102: 276-84
- BROWN C, JOSHI B, FARADAY N, et al. Emergency cardiac surgery in patients with acute coronary syndromes: a review of the evidence and perioperative implications of medical and mechanical therapeutics. Anesth Analg 2011; 112: 277-99
- CHANDLER WL, VELAN T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003; 101:4355-62
- CHANDLER WL, VELAN T. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coag Fibrinolysis 2004; 15:583-91
- COPPENS M, EIKELBOOM JW, GUSTAFSSON D, WEITZ JI. Translational success stories. Development of direct thrombin inhibitors. Circ Res 2012; 111:920-9
- Czerny M, Baumer H, Kilo J, et al. Inflammatory response and myocardial injury following coronary artery bypass graTFing with or without cardiopulmonary bypass. Eur J Cardiothorac Surg 2000; 17: 737-42
- DE FOE GR, ROSS CS, OLMSTEAD EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass graTFing. Ann Thorac Surg 2001; 71:769-76
- Diegeler A, Doll N, Rauch T, et al. Humoral immune response during coronary artery bypass graTFing: A comparison of limited approach, "off-pump" technique, and conventional cardiopulmonary bypass. Circulation 2000; 102: III95-100
- DIETRICH W, BUSLEY R, SPANNAGL M, et al. The influence of antithrombin substitution on heparin sensitivity and activation of hemostasis during coronary artery bypass graTF surgery: a dose-finding study. Anesth Analg 2013; 116 :1223-30
- DUNNING J, VERSTEEGH M, FABBRI A, et al. Guidelines on antiplatelet and anticoagulation management in cardiac surgery. Eur J Cardiothorac Surg 2008; 34:73-92
- FINLEY A, GREENBERG C. Heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg 2013; 116:1210-22
- FONTES ML, MATHEW JP, RINDER HM, et al. Atrial fibrillationaftercardiopulmonary bypass is associated with monocyte activation. Anesth Analg 2005; 101:17-23
- FRANZ A, BRAUNLICH P, et al. The effects of hydroxyethyl starches of varying molecular weights on platelet function. Anesth Analg 2001; 92: 1402-7
- GARCIA DA, BAGLIN TP, WEITZ JI, et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e24S-e43S
- GHADIMI K, LEVY JH, WELSBY IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg 2016; 122:1287-300
- GORMLEY SMC, McBRIDE WT, ARMSTRONG MA, et al. Plasma and urinary cytokine homeostasis and renal function during cardiac surgery without cardiopulmonary bypass. Cytokine 2002; 17:61-5
- GRONCHI F, RANUCCI M. Perioperative coagulation in cardiovascular surgery. In: MARCUCCI C, SCHOETKER P, editors. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer Verlag, 2014, 243-66
- HAASE M, HAASE-FIELITZ A, BELLOMO R, et al. Sodium bicarbonate to prevent increases in serum creatinineaftercardiac surgery: a pilot double-blind, randomized controlled trial. Crit Care Med 2009; 37:39-47
- HABIB RH, ZACHARIAS A, SCHWANN TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg 2003; 125:1438-50
- HALL TS. The pathophysiology of cardiopulmonary bypass: The risks and benefits of hemodilution. Chest 1995: 100:88-94
- HENNEIN HA. Inflammationaftercardiopulmonary bypass: therapy for the postpump syndrome. Semin Cardiothorac Vasc Anesth 2001; 5:236-55
- JONAS RA, WYPIJ D, ROTH SJ, et al. The influence of hemodilution on outcomeafterhypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 2003; 126:1765-74
- KELTON JG, ARNOLD DM, BATES SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med 2013; 368:737-44
- KOSTER A, DYKE CM, ALDEA G, et al. Bivalirudin during cardiopulmonary bypass in patients with previous or acute heparin-induced thrombocytopenia and heparin antibodies: Results of the CHOOSE-ON trial. Ann Thorac Surg 2007; 83:572-7
- KOSTER A, FARAONI D, LEVY JH. Argatroban and bivalirubin for perioperative anticoagulation in cardiac surgery. Anesthesiology 2018; 128:390-400
- KRAJEWSKI S, KURZ J, NEUMANN B, et al. Short-acting P2Y12 blockade to reduce platelet dysfunction and coagulopathy during experimental extracorporeal circulation and hypothermia. Br J Anaesth 2012; 108:912-21
- Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology 2002; 97: 215-52
- LEVY JH, MONTES F, SZLAM F, et al. The in vitro effect of antithrombin III on the activated coagulation time in patients on heparin therapy. Anesth Analg 2000; 90: 1076-9
- LIAM BL, PLOCHL W, COOK DJ, et al. Hemodilution and whole body balance during normothermic cardiopulmonary bypass. J Cardiothorac vasc Surg 1998; 115:1203-8
- MANGOUSH O, PURKAYASTHA S, HAJ-YAHIA S, et al. Heparin-bonded circuits versus nonheparin-bonded circuits: an evaluation of their effect on clinical outcomes. Eur J Cardiothorac Surg 2007; 31:1058-69
- Menasche P. The systemic factor: the comparative roles of cardiopulmonary bypass and off-pump surgery in the genesis of patient injury during and following cardiac surgery. Ann Thorac Surg 2001; 72: S2260-5; discussion S5-6
- MONGERO LB, BECK JR (eds). On bypass. Advanced perfusion techniques. Policy and procedure guidelines (CP36): Cold agglutinins. Totowa (NJ, USA): Humana Press 2010, 426-8
- Nathoe HM, Van Dijk D, Jansen EWL, et al. A comparison of on-pump and off-pump coronary ECC in low-risk patients. N Engl J Med 2003; 348:394-402
- OHRI SK, BOWLES CW, MATHIE RT, et al. Effect of cardiopulmonary bypass perfusion protocols on gut tissue oxygenation and blood flow. Ann Thorac Surg 1997; 64:163-8
- Puskas JD, Williams WH, Duke PG, et al. Off-pump coronary artery bypass graTFing provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: A prospective randomized comparison of two-hundred unselected patients undergoing off-pump versus conventional coronary artery ECC. J Thorac Cardiovasc Surg 2003; 125:797-808
- RANUCCI M, BALDUINI A, DITTA A; et al. A systematic review of biocompatible cardiopulmonary bypass circuits and clinical outcome. Ann Thorac Surg 2009; 87: 1311-9
- RINDER C. Cellular inflammatory response and clinical outcome in cardiac surgery. Curr Opin Anaesthesiol 2006; 19:65-8
- ROZENTAL T, SHORE-LESSERSON L. Pharmacologic management of coagulopathy in cardiac surgery: An update. J Cardiothorac Vasc Anesth 2012; 26:660-79
- Sharony R, Bizekis CS, Kanchuger M, et al. Off-pump coronary artery bypass graTFing reduces mortality and stroke in patients with atheromatous aortas: A case control study. Circulation 2003; 108 (suppl II):15-20
- SNIECINSKI RM, CHANDLER WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113:1319-33
- SUNGURTEKIN H, COOK DJ, ORSZULAK TA, et al. Cerebral response to hemodilution during hypothermic cardiopulmonary bypass in adults. Anesth Analg 1999; 89:1078-83
- SWAMINATHAN M, PHILIPS-BUTE BG, CONLON PJ, et al. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injuryaftercoronary artery ECC. Ann Thorac Surg 2003; 76:784-91
- UNTCH BR, JESKE WP, SCHWARTZ J, et al. Inflammatory and hemostatic activation in patients undergoing off-pump coronary artery bypass graTFing. Clin Appl Thromb Hemost 2008; 14-141-8
- VAN WERMESKERKEN GK, LARDENOYE JWH, HILL SE, et al. Intraoperative physiologic variables and outcome in cardiac surgery. Part II. Neurologic outcome. Ann Thorac Surg 2000; 69:1077-83
- WANG G, BAINBRIDGE D, MARTIN J, et al. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg 2009; 109:320-30
- WEBER CF, KLAGES M, ZACHAROWSKI K. Perioperative coagulation management during cardiac surgery. Curr Opin Anaesthesiol 2013; 26: 60-84
- WILLIAMS MR, D'AMBRA AB, BECK JR, et al. A randomized trial of antithrombin concentrate for treatment of heparin resistance. Ann Thorac Surg 2000; 70:873-7