In addition to coarctation, various anomalies may occur in the aortic arch: hypoplasia, patent ductus arteriosus, aortopulmonary fistulas, coronary artery anomalies.
Coronary artery anomalies
Multiple anatomical variations of the coronary vascular tree exist, occurring in 0.3% of the population (Figure 15.60) [2].
Coronary artery anomalies
Multiple anatomical variations of the coronary vascular tree exist, occurring in 0.3% of the population (Figure 15.60) [2].

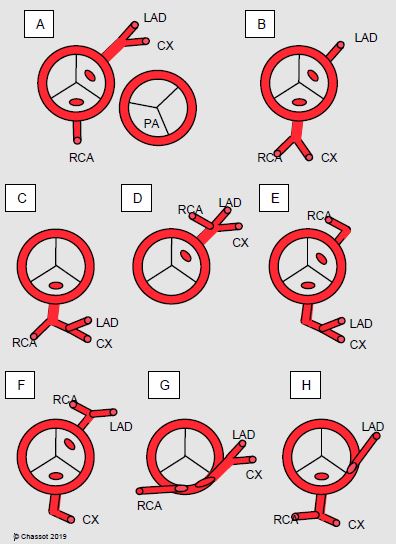
Figure 15.60: Diagram of the most common coronary artery anomalies. A: normal situation with the position of the pulmonary valve (PA). B to H: examples of anomalies. In B, C, E, F and H, a vessel crosses the front of the PA and may cause problems during surgery of the right ventricular outflow tract. G: intramural position [2]. LAD: left anterior descending artery. CX: circonflex artery. RCA: right coronary artery.
Some are benign, while others may cause arrhythmias, sudden death, or coronary ischaemia. The most dangerous examples are insertion of the left main trunk in the right sinus of Valsalva or insertion of the dominant right coronary artery in the left sinus of Valsalva, high insertion in the aorta, intramyocardial coursing, single ostium for the three trunks and coursing between the aorta and PA [3]. Coronary artery anomalies may also cause major surgical issues, especially if the LAD crosses the front of the RVOT in a correction of tetralogy of Fallot or pulmonary stenosis. A coronary artery may also dilate and cause an aneurysm, which is in some cases massive (Figure 27.18F). In ALPACA syndrome (anomalous left coronary artery from the pulmonary artery), the insertion of the coronary artery in the pulmonary artery compromises myocardial perfusion of the affected area and requires early surgical revascularisation.
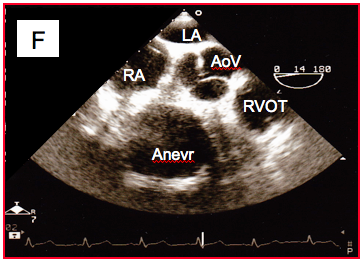
Figure 27.18F: Aneurysm located on the main part of the right coronary artery (transesophageal basal view at the level of the aortic valve (AoV).
A coronary artery may originate abnormally from the aorta or terminate in the RV or PA, creating a left-to-right fistula. This results in significant shunting of approximately ≥ 1/1.6, and correction is necessary [4]. The surgical correction in indicated in case of myocardial ischaemia or L-to-R fistula (ALPACA syndrome) [7].
Patent ductus arteriosus
The patent ductus arteriosus connects the descending aorta just after the left subclavian artery root to the root of the left pulmonary artery (see Figure 14.71). Some patients keep a patent ductus arteriosus until adulthood. It causes L-to-R shunting, overloading the LV and potentially resulting in its failure. The shunt's flow rate is dependent on the size of the ductus arteriosus and the ratio of systemic to pulmonary resistance (SVR/PVR). If the ductus arteriosus is narrow, L-to-R shunting becomes symptomatic around the age of thirty years due to LV volume overload. Diagnosis is established on auscultation: continuous 4/6 murmur with a systolic crescendo at the top of the sternum radiating to the back. Echocardiography reveals a continuous turbulent flow suspended in the pulmonary artery and a concentric acceleration zone in the aorta at the entry to the ductus arteriosus. These flows are mainly visible during diastole [5]. Patency of a large-diameter ductus arteriosus causes LV dilation and pulmonary hypertension. This leads secondarily to RV hypertrophy and failure. If PAH reaches similar pressure levels to those in the aorta, shunting becomes bidirectional even if pulmonary diastolic pressure remains lower than systemic diastolic pressure for a long period.
Surgery is not indicated for a small silent patent ductus arteriosus [6]. It is indicated if the murmur is audible, if the LV is subjected to volume overload, or if the patient has a history of endocarditis. Shunting must be exclusively L-to-R and the PVR/SVR ratio must be under 0.65. If shunting is bidirectional, banding is the only possible means of reducing the flow [6,7]. The procedure is increasingly performed percutaneously (transcatheter coil closure) or by thoracoscopy. Ligation of the patent ductus arteriosus by thoracotomy is currently only indicated for cases where the patent ductus arteriosus is too wide, calcified or twisted to be closed via the endovascular route [1,8].
Intraoperative monitoring of SpO2 in the right hand and right foot is beneficial. At constant SpO2 in the upper limb, variations of SpO2 in the foot are inversely proportionate to blood flow through the patent ductus arteriosus. In cases of bidirectional shunting, SpO2 in the foot is significantly lower than in the hand as the shunt flows partially R-to-L. Arterial vasodilation reduces the shunt, provided that PAP is significantly lower than systemic pressure. However, if the two pressures are similar and shunting is bidirectional, steps must be taken to prevent any drop in systemic pressure as this would increase the R-to-L component of the shunt and exacerbate post-ductal cyanosis. Ligation of the patent ductus arteriosus immediately prompts a rise in systemic diastolic pressure, which was previously low. It also causes circulating volume overload since volume that had previously been diverted to the lungs is reintroduced into the circulation.
Aortopulmonary fistula
A small communication between the ascending aorta and the trunk of the pulmonary artery (aortopulmonary fistula) is occasionally encountered in adults. It creates a L-to-R shunt similar to that observed in patent ductus arteriosus. The size of the fistula determines the size of this shunt and the degree of volume overload for the LV. It appears on an echocardiogram as a small jet in the right anterolateral side of the PA trunk, which is mainly visible during diastole as it is concealed by the PA flow during systole. It is treated by surgical closure.
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
Some are benign, while others may cause arrhythmias, sudden death, or coronary ischaemia. The most dangerous examples are insertion of the left main trunk in the right sinus of Valsalva or insertion of the dominant right coronary artery in the left sinus of Valsalva, high insertion in the aorta, intramyocardial coursing, single ostium for the three trunks and coursing between the aorta and PA [3]. Coronary artery anomalies may also cause major surgical issues, especially if the LAD crosses the front of the RVOT in a correction of tetralogy of Fallot or pulmonary stenosis. A coronary artery may also dilate and cause an aneurysm, which is in some cases massive (Figure 27.18F). In ALPACA syndrome (anomalous left coronary artery from the pulmonary artery), the insertion of the coronary artery in the pulmonary artery compromises myocardial perfusion of the affected area and requires early surgical revascularisation.

Figure 27.18F: Aneurysm located on the main part of the right coronary artery (transesophageal basal view at the level of the aortic valve (AoV).
A coronary artery may originate abnormally from the aorta or terminate in the RV or PA, creating a left-to-right fistula. This results in significant shunting of approximately ≥ 1/1.6, and correction is necessary [4]. The surgical correction in indicated in case of myocardial ischaemia or L-to-R fistula (ALPACA syndrome) [7].
Patent ductus arteriosus
The patent ductus arteriosus connects the descending aorta just after the left subclavian artery root to the root of the left pulmonary artery (see Figure 14.71). Some patients keep a patent ductus arteriosus until adulthood. It causes L-to-R shunting, overloading the LV and potentially resulting in its failure. The shunt's flow rate is dependent on the size of the ductus arteriosus and the ratio of systemic to pulmonary resistance (SVR/PVR). If the ductus arteriosus is narrow, L-to-R shunting becomes symptomatic around the age of thirty years due to LV volume overload. Diagnosis is established on auscultation: continuous 4/6 murmur with a systolic crescendo at the top of the sternum radiating to the back. Echocardiography reveals a continuous turbulent flow suspended in the pulmonary artery and a concentric acceleration zone in the aorta at the entry to the ductus arteriosus. These flows are mainly visible during diastole [5]. Patency of a large-diameter ductus arteriosus causes LV dilation and pulmonary hypertension. This leads secondarily to RV hypertrophy and failure. If PAH reaches similar pressure levels to those in the aorta, shunting becomes bidirectional even if pulmonary diastolic pressure remains lower than systemic diastolic pressure for a long period.
Surgery is not indicated for a small silent patent ductus arteriosus [6]. It is indicated if the murmur is audible, if the LV is subjected to volume overload, or if the patient has a history of endocarditis. Shunting must be exclusively L-to-R and the PVR/SVR ratio must be under 0.65. If shunting is bidirectional, banding is the only possible means of reducing the flow [6,7]. The procedure is increasingly performed percutaneously (transcatheter coil closure) or by thoracoscopy. Ligation of the patent ductus arteriosus by thoracotomy is currently only indicated for cases where the patent ductus arteriosus is too wide, calcified or twisted to be closed via the endovascular route [1,8].
Intraoperative monitoring of SpO2 in the right hand and right foot is beneficial. At constant SpO2 in the upper limb, variations of SpO2 in the foot are inversely proportionate to blood flow through the patent ductus arteriosus. In cases of bidirectional shunting, SpO2 in the foot is significantly lower than in the hand as the shunt flows partially R-to-L. Arterial vasodilation reduces the shunt, provided that PAP is significantly lower than systemic pressure. However, if the two pressures are similar and shunting is bidirectional, steps must be taken to prevent any drop in systemic pressure as this would increase the R-to-L component of the shunt and exacerbate post-ductal cyanosis. Ligation of the patent ductus arteriosus immediately prompts a rise in systemic diastolic pressure, which was previously low. It also causes circulating volume overload since volume that had previously been diverted to the lungs is reintroduced into the circulation.
Aortopulmonary fistula
A small communication between the ascending aorta and the trunk of the pulmonary artery (aortopulmonary fistula) is occasionally encountered in adults. It creates a L-to-R shunt similar to that observed in patent ductus arteriosus. The size of the fistula determines the size of this shunt and the degree of volume overload for the LV. It appears on an echocardiogram as a small jet in the right anterolateral side of the PA trunk, which is mainly visible during diastole as it is concealed by the PA flow during systole. It is treated by surgical closure.
| Arterial abnormalities |
|
Some coronary artery anomalies cause major arrhythmias, sudden death or myocardial ischaemia.
The patent ductus arteriosus is a communication between the descending aorta just after the left subclavian artery root and the left pulmonary artery root. It causes L-to-R shunting. The patent ductus arteriosus is closed by endovascular or surgical route if it causes shunting that overloads the LV, provided that the PVR/SVR ratio is < 0.7. If this ratio is close to 1, shunting is bidirectional and can no longer be occluded. |
© BETTEX D, CHASSOT PG, January 2008, last update February 2020
References
- BAUMGARTNER H, BONHOEFFER P, DE GROOT NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915-57
- BETTEX D, CHASSOT PG. Transesophageal echocardiography in congenital heart disease. In: BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1186-1212
- BHATT AB, FOSTER E, KUEHL K, et al. Congenital hesart disease in older adult. A Scientific Statement from the American Heart Association. Circulation 2015; 131:1884-931
- BISHOP A. Coronary artery anomalies. In: REDINGTON A, et al. ed. Congenital heart disease in adults. A practical guide. London, WB Saunders Co Ltd, 1994, pp 153-60
- MILLER-HANCE WC, SILVERMAN NH. Transesophageal echocardiography (TEE) in congenital heart disease with focus on the adult. Cardiol Clinics 2000; 18:861-92
- SILVERSIDES CK, DORE A, POIRIER N, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Shunt lesions. Can J Cardiol 2010; 26:e70-e79
- STOUT KK, DANIELS CJ, VALENTE AM, et al. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease. J Am Coll Cardiol 2019; 73:e81-192
- WARNES CA, WILLIAMS RG, BASHORE TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008; 118:2395-451
