Ventricular dysfunction is common in congenital diseases. It is linked to several phenomena.
- Ventricular remodelling due to heart defects: e.g. right-side failure of a subaortic RV functioning as a systemic ventricle, spherization of a single ventricle (SV). Function is improved if the SV is anatomically left-sided. Diastolic dysfunction with reduced compliance is common and requires above-average filling pressures.
- The duration of the malformation: the later the surgical correction is performed, the greater the degree of remodelling. On the other hand, palliative operations do not protect the ventricles to the same extent as total corrections.
- The type of overload: volume overload is better tolerated than pressure overload. The latter leads to concentric hypertrophy, excessive wall tension, inadequate coronary blood flow and low tolerance to an imbalance in the DO2/VO2 ratio. In an L-to-R shunt, the RV fails if pulmonary systolic pressure rises above 50 mmHg or 50% of the systemic pressure [11]. A volume overload causes dilated hypertrophy, which can cope with an increase in end-diastolic volume of around 40%. However, the efficiency of this ventricle is diminished, as it is working over a large diameter (LaPlace’s law) and becomes very sensitive to any increase in afterload.
- Cyanosis: myocardial VO2 exceeds the DO2 upon the slightest exertion and episodes of hypoxic dysfunction are common. Reduced myocardial perfusion pressure due to the shunt and microvascular occlusions caused by hyperviscosity lead to chronic ischemia, which results in irreversible lesions (fibrosis).
- Ischemia: possibly due to malformations, surgical corrections, cyanosis, or microvascular occlusions caused by hyperviscosity. In cases of pulmonary arterial hypertension (PAH), it threatens the RV as soon as the systemic pressure drops.
Right ventricle
In patients with congenital heart diseases, heart failure most commonly affects the right ventricle. Volume overload (ASD, tricuspid insufficiency or major pulmonary insufficiency) causes an eccentric hypertrophy and dilatation of the RV. It is well tolerated for a long time, but entails a high risk of refractory ventricular arrhythmia [2]. A chronic increase in afterload from birth (pulmonary stenosis, PAH, RV in systemic position) causes concentric hypertrophy rather than RV dilation. Right heart function remains adequate as long as intraventricular pressure is < 50% of the left heart pressure and there is no associated volume overload (as occurs during tricuspid insufficiency). Patients become symptomatic if the pressure of the RV exceeds half of the systemic pressure [1]. Pharmacological treatment of right ventricular failure is largely ineffective for tackling congenital diseases (see Table 14.8) [3]
When subjected from birth to a high afterload (pulmonary hypertension, systemic ventricle), the RV retains its foetal configuration, does not thin, and keeps the same wall thickness as the LV. It may function in this way for several decades. As a result, the prognosis for congenital pulmonary hypertension (PHT) is much better than for PHT occurring later in adult life, since it is dependent on right heart function [7]. The performance of the RV is highly dependent on that of the LV because the latter contributes > 40% to right-side ejection by the contraction of the interventricular septum, which belongs physiologically to the LV. This contribution is lost in the case of a wide VSD, left-side hypoplasia or significant remodelling of the left-side chambers [5]. On the other hand, RV dilation through volume overload, as occurs with a wide ASD, pushes the septum back inside the LV during diastole and restricts the filling of the left heart. If PHT is added to this situation, septal swing also takes place during systole. In addition, the duration of RV systolic ejection is extended if right-side afterload is high, and the peaks in systolic pressure between the two ventricles are desynchronised. In this situation, a rise in left-side afterload has two benefits: it increases LV contractility by Anrep effect, and places the septum back in a position that is curved to the right by increasing left-sided intraventricular pressure. Correcting the position of the septum also decreases the dilation of the tricuspid annulus and reduces the degree of tricuspid insufficiency [5]. Hence, systemic vasoconstriction (increasing SVR) is an effective therapy for right-sided heart failure.
The risk of right ventricular myocardial ischemia increases as PAP rises. Indeed, the coronary perfusion of the RV is systolic and diastolic, while it is mainly diastolic for the LV (Figure 14.13).
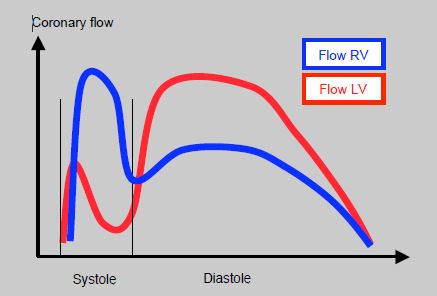
Although systemic diastolic pressure remains higher than pulmonary diastolic pressure, the approximation of the two systolic pressures in the case of severe PHT reduces the coronary perfusion pressure of the RV by reducing its systolic component. With almost half of its O2 supply removed, right-side coronary perfusion predominates during diastole and is therefore similar to that of the LV [2]. Right-side coronary perfusion is improved by an increase in systemic pressure with vasoconstrictors.
In infants, the symptoms of congestive ventricular failure include feeding difficulties and delayed growth, and, in severe cases, apathy and a weak cry. Irritability, tachycardia, extreme inspiratory effort (intercostal retraction, flaring of the nasal alae) and hepatomegaly are also observed [4].
Diagnostic methods
Echocardiography is the simplest and least invasive examination, and is therefore given priority. In addition, transthoracic windows offer high-quality images in children. A 2D echocardiography provides information on basic anatomy, heart chamber morphology and function, valves and their functioning. The Doppler measures blood flow, gradients, shunts and, indirectly, RV and PA pressure. The relatively simple shape of the LV allows for a fairly accurate quantitative assessment of its function by means of geometric approximations that allow its ejection fraction (EF) to be calculated. However, the EF is not a satisfactory criterion of systolic function in congenital heart diseases due to significant morphological changes of the ventricles and their very specific preload or afterload conditions. Systolic and diastolic dimension measurements offer the best means of assessment. The situation is even more difficult for the RV, whose complex morphology and significant remodelling exclude the use of geometric approximations that are applicable for the LV. Measurement of the acceleration (dP/dt) of isovolumetric contraction and assessment of local deformation (strain and strain rate) are more accurate techniques, less dependent on the shape of the RV and its loading conditions [12,14]. Measurement of the movement of the tricuspid annulus (TAPSE tricuspid annular plane systolic excursion), considered to be an excellent reflection of the longitudinal contraction of the RV in adults, correlates poorly with ventricular performance in children whose afterload is abnormal as in pulmonary stenosis or systemic RV, probably because of the predominant circular musculature in this context [8]. A 3D echocardiography offers greater accuracy in the assessment of the RV, although it tends to underestimate RV volume compared to MRI [1].
MRI provides a very detailed three-dimensional reconstruction of the anatomy and is far superior to an echocardiogram in terms of assessing the RV, chamber volume, tissue characteristics (viability) and extracardiac vessels (pulmonary veins, aorta, PA, tributaries). However, it requires 10-20 second periods of apnoea to acquire images, a regular and not excessively fast heart rate, and a lack of movement that is difficult to achieve in children without deep sedation or general anaesthesia [9]. Multislice CT angiography offers the best spatial resolution of structures, and particularly extracardiac structures (vessels, shunts, coronary arteries), but has the disadvantage of using ionising radiation (40-70 mGy) and contrast agents. The new generation of dual-source and 128-slice equipment limits irradiation and the need for apnoea [6]. The CT scan also requires the subject to have quite a low heart rate and remain still.
MRI and CT scans are not risk-free as infants require anaesthesia. As the sedation must be deep in order to ensure that infants remain still, the danger of hypoventilation and hypercapnia is excessive in those who suffer from pulmonary hypertension or are at risk of PHT episodes. As a result, general anaesthesia with intubation and controlled ventilation is usually preferable under 6 years of age: induction with sevoflurane, maintenance with sevoflurane or propofol (1-2 mg/kg/h), curarisation (rocuronium or vecuronium). Periods of apnoea are managed by simply disconnecting one limb of the circle circuit from the ventilator, which can even be done from the control station if the system has an extra-long circle [13]. Indeed, the complication rate varies from 8% to 28%. The main complications are episodes of hypotension and bradycardia. However, ventricular decompensation and cardiac arrest are also observed. Fasting must be limited and hydration must be ensured by an intravenous infusion (10-20 mL/kg for the duration of the examination) given the risks of hypotension and shunt thrombosis [10,13]. Post-operative monitoring must be continued until the next day in 10-25% of cases, depending on clinical condition and pathology [6].
| Ventricular dysfunction |
|
The ventricular function of congenital heart disease patients can never be considered as normal, excluding those with ASDs, small VSDs and patent ductus arteriosus operated on in infancy. The lack of reserve, dysfunction, or ventricular failure are linked to several phenomena:
- Anatomical remodelling - Palliative operation - Subaortic (systemic) RV, single ventricle - Prolonged duration of life before correction - Pressure overload, less well tolerated than volume overload - Cyanosis - Chronic ischemia Ventricular failure occurs most commonly in the RV, because of a volume overload (L-to-R shunt) or pressure overload (PAH, pulmonary stenosis, systemic RV, single ventricle). Increasing the afterload of the LV (↑ SVR) repositions the interventricular septum so that it can contribute to the RV ejection. Echocardiography is the preferred choice of examination for ventricular function. MRI is more effective for RV function, extracardiac vessels, and assessing chamber volume. Infants require deep sedation or a GA in order to undergo MRI and CT scans. |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
- CREAN AM, MAREDIA N, BALLARD G, et al. 3D echo systematically underestimates right ventricular volumes compared to cardiovascular magnetic resonance in adult congenital heart disease patients with moderate or severe right ventricular dilatation. J Cardiovasc Magn Resn 2011; 13:78
- CRYSTAL GJ, PAGEL PS. Right ventricular perfusion. Physiology and clinical implications. Anesthesiology 2018; 128:202-18
- DAVLOUROS PA, NIWA K, WEBB G, GATZOULIS MA. The right ventricle in congenital heart disease. Heart 2006; 92(Suppl 1): i27-i38
- DUPUIS C, KACHANER J, FREEDOM R, PAYOT M, DAVIGNON A. Cardiologie pédiatrique, 2ème édition. Paris: Flammarion, 1991, 115-123
- FRIEDBERG MK, REDINGTON MB. Right versus left ventricular failure. Differences, similarities, and interactions. Circulation 2014; 129:1033-44
- GOTTLIEB EA, ANDROPOULOS D. Anesthesia for the patient with congenital heart disease presenting for noncardiac surgery. Curr Opin Anesthesiol 2013; 26:318-26
- HOPKINS WE. The remarkable right ventricle of patients with Eisenmenger syndrome. Coron Artery Dis 2005; 16:19-25
- KOESTENBERGER M, NAGEL B, RAVEKES W, et al. Tricuspid annular plane systolic excursion and right ventricle ejection fraction in pediatric and adolescent patients with tetralogy of Fallot, patients with atrial septal defect, and age-matched normal subjects. Clin Res Cardiol 2011; 100:67-75
- NTSINJANA HN, HUGHES ML, TAYLOR AM. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J Cardiovasc Magn Reson 2011; 13:51-70
- ODEGARD KC, VINCENT R, BAIJAL RG, et al. SCAI/CCAS/SPA Expert Consensus Statement for anesthesia and sedation practice: recommendations for patients undergoing diagnostic and therapeutic procedures in the pediatric and congenital cardiac catheterization laboratory. Anesth Analg 2016; 123:1201-9
- PETTERSEN E, HELLE-VALLE T, EDVARDSEN T, et al. Contraction pattern of the systemic right ventricle. J Am Coll cardiol 2007; 49:2450-6
- SHERPTONG RWC, MOLLEMA SA, BLOM NA, et al. Right ventricular peak systolic longitudinal strain is a sensitive marker for right ventricular deterioration in adult patients with tetralogy of Fallot. Int J Cardiovasc Imag 2009; 25:669-76
- STOCKTON E, HUGHES M, BROADHEAD M, et al. A prospective audit of safety issues associated with general anesthesia for pediatric cardiac magnetic resonance imaging. Pediatr Anesth 2012; 22:1087-93
- TOYONO M, HARADA K, TAMURA M, et al. Myocardial acceleration during isovolumic contraction as a new index of right ventricular contractile function and its relation to pulmonary regurgitation in patients after repair of tetralogy of Fallot. J Am Soc Echocardiogr 2004; 17:332-7