When anticoagulation is provided with a long-acting agent and cannot be interrupted, as in high-risk AF or mechanical valve replacement, it is usual to relay treatment with a short half-life heparin to limit off time anticoagulant effect to the duration of the surgery. The same remarks made for reversal apply here: the aim is not the normalisation of coagulation, which would present a major danger of vascular accident, but the best compromise between the surgical haemorrhagic risk and the thrombo-embolic risk of the disease. The main reason for substitution is the higher mortality in venous thrombosis (pulmonary embolism) or arterial thrombosis (infarction, stroke) than in major bleeding. Although traditionally considered a best practice, heparin substitution is currently being questioned, particularly for newer anticoagulants [1,7,17]. Indeed, recent studies show that it increases the risk of bleeding 3 to 5 times without protecting against thrombotic complications (unchanged incidence). A meta-analysis of 7,118 patients on AVKs who were substituted with heparin preoperatively shows that risk of minor and major bleeding is increased (HR 5.4 and 3.6, respectively), while the thrombo-embolic risk is unchanged (HR 0.84) [14]. The rate of haematoma may even be higher with substitution than with continued baseline therapy [3]. A prospective registry of 2179 patients on NOACs (rivaroxaban, apixaban and dabigatran) found a similar incidence of thromboembolic events in substituted (1.6%) and non-substituted (0.8%) patients, but a fivefold greater blood loss in substituted patients (2.7%) compared to non-substituted patients (0.5%) (OR 5.0) [2]. With very strict selective criteria for study selection, a review shows that discontinuation of AVKs does not increase thromboembolism risk (RR 0.65) but decreases bleeding risk (RR 0.41); moreover, substitution appears to unsettle anticoagulant therapy [11].
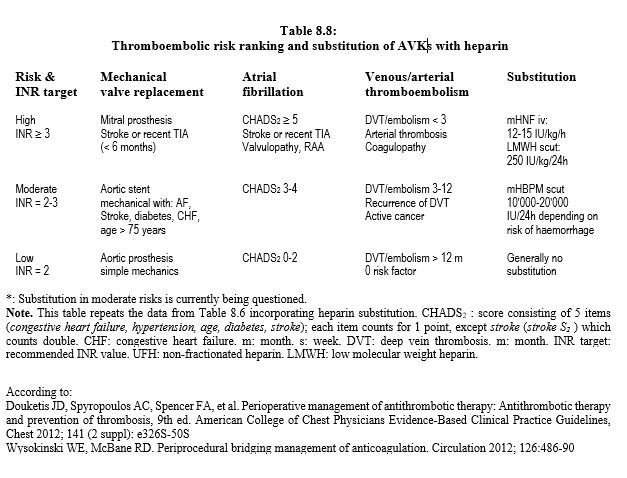
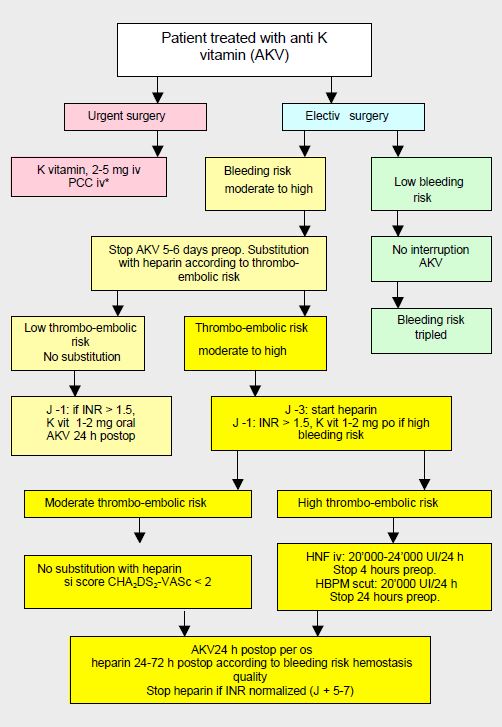
In the current state of knowledge, substitution is therefore only formally indicated in patients at very high risk of thrombosis (high CHADS score, mechanical heart valve prosthesis, history of embolic stroke) who are on AVKs. In other situations, it probably offers no benefit and is a case-by-case decision. Apart from the very high thrombotic risks when interrupted for >96 hours, it is not justified for NOACs, as it increases the risk of bleeding (HR 3.5) without reducing the risk of stroke or thromboembolism [2,4,7,13]. As much of bleeding occurs postoperatively, it is recommended that replacement anticoagulation is not restarted for 24-72 hours, depending on haemostasis quality [16]. The perioperative management of patients on AVK can be represented by an algorithm (Figure 8.15) [5,6,9]. It is based on a stratification of thromboembolic risk into 3 categories as described in Table 8.8.
- Minor or non-haemorrhagic operations (dentistry, skin excision, cataract, biopsy, endoscopy, pacemaker, etc) can be performed without interruption of AVKs, although bleeding rate is 3 times higher. Retrobulbar block has a risk of haematoma of only 0.7% on AVKs [12]. Perioperatively, the target INR is at the lower limit of the therapeutic range.
- Stop AVKs 5-6 days before procedures with intermediate or major bleeding risk (Marcoumar® : stop 10 days).
- Substitution with a heparin 3 days before the operation depending on the thrombo-embolic risk.
- Cases with high thromboembolic risk: UFH 12-15 IU/kg/h (20,000-30,000 IU/24 h) infusion, no bolus; stop 4-6 hours before surgery. Alternative: LMWH at therapeutic dose (250 IU/kg/24 h, ≥ 20,000 IU/24 h) in 2 daily subcutaneous doses; stop 24 hours before surgery; if bleeding risk is high, the last administration (24 h pre-op) is a half-dose
- Cases with moderate thromboembolic risk: LMWH at therapeutic dose (250 IU/kg/24h) in 2 daily subcutaneous doses; stop 24 hours before the procedure. Alternative in cases with high bleeding risk: LMWH prophylactic dose (10,000 IU/24h subcutaneous); stopped 12-24 hours before the procedure. In this indication, substitution is no longer recommended: in CHADS2 2-3, it does not alter the thrombotic risk compared to simple discontinuation, but increases the risk of bleeding [4,8].
- Cases with low thromboembolic risk: no substitution.
- Preference for subcutaneous LMWH whenever possible, unless creatinine clearance is < 30 mL/min, when iv UFH is recommended.
- Intravenous administration requires hospitalisation, while the subcutaneous form allows treatment at home.
- Patients with a history of HIT and anti-heparin antibodies: bivalirudin or argatroban.
- Interruption of heparin 4 hours (intravenous UFH) to 12-24 hours (LMWH) pre-op.
- High embolic risk (< 1 monthaftervenous thrombosis of the lower limbs or pelvis) and emergency operation (substitution impossible): placement of a removable filter on the inferior vena cava.
- Resume AVKs 12-24 hours postoperatively (evening or day after surgery).
- Resume heparin within 24-48 hours postoperatively for 4-6 days; this can be extended to 72 hours if the risk of bleeding is very high; resumption at less than 24 hours doubles the risk of postoperative bleeding but is preferable in cases of high thromboembolic risk [15]. Heparin is stopped only when the INR is back in the therapeutic range.
New oral anticoagulants (dabigatran, rivaroxaban, apixaban, edoxaban) have half-lives of about 12 hours requiring a 48-hour interruption, or 3-5 days in the elderly and in patients with renal failure. Their antidotes are not yet available, except for dabigatran. Generally, there is no need to start a relay with heparin for these substances, except in cases with very high risk of thrombosis such as mechanical valve prostheses, but the latter are preferably on AVK [17]. Since the pharmacokinetics of NOACs are similar to those of LMWHs, substitution with LMWHs makes no sense and has no indication [2,10,13]. In case of doubt in the preoperative setting, the best attitude is to assay the anticoagulant (calibrated anti-Xa effect for the substance for rivaroxaban, edoxaban and apixaban, anti-IIa effect or Hemoclot™ for dabigatran). If the level is high, substitution is not necessary; if it is low, there is no need to wait and the patient is to be operated without delay. During postoperative recovery in cases of high bleeding risk and high thrombo-embolic risk, it is possible to start anticoagulant treatment with non-fractionated heparin for which protamine is an effective antidote and always available. The NOAC is resumed when the situation is stabilised, taking care that the two substances are never administered simultaneously.
Figure 8.15: Algorithm for the management of patients on anti-vitamin K agents (AVKs) preoperatively in non-cardiac surgery, based on surgical bleeding risk and thromboembolic disease risk [adapted from references [9,17]. Heparin replacement is no longer recommended when the thromboembolic risk is moderate (CHA score2 DS2 -VASc ≤ 2). PCC: prothrombin complex concentrate. PCC: prothrombin complex concentrate *: according to blood loss and not as a prophylaxis.
| Perioperative substitution of anticoagulants |
|
Substitution of AVKs with heparin is reserved for cases with a high thrombo-embolic risk. In other situations, it increases the risk of bleeding 3 to 5 times without reducing the thromboembolic risk. It is not recommended for procedures with high bleeding risk or for patients with low thromboembolic risk. It is not recommended with new oral anticoagulants (dabigatran, rivaroxaban, apixaban, edoxaban). In situations of high thromboembolic risk (recent deep vein thrombosis, AF CHADS2 > 4, mechanical mitral prosthesis), the AVK is replaced by a short half-life heparin. The aim is not to normalise coagulation, which would present a major risk of vascular accident, but to find the best compromise between bleeding risk during surgery and thrombo-embolic risk for that disease.
Substitution of AVKs :
- Stop AVK 5 days pre-op (Marcoumar 10 d)
- Substitution with heparin 3 days before the operation
- Interruption of heparin 4-6 hours (UFH iv) to 24 hours (LMWH scut) preoperatively
- Resumption of AVKs at ≤ 24 hours postoperatively
- Resume heparin 12-48 hours post-operatively, stopped when INR is within
therapeutic values
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- BARON TH, KAMATH PS, McBANE RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med 2013; 368:2113-24
- BEYER-WESTENDORF J, GELBRICHT V, FORSTER K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from prospective Dresden NOAC registry. Eur Heart J 2014; 35:1888-96
- BIRNIE DH, HEALEY JS, WELLS GA, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368 :2084-93
- DOHERTY JU, GLUCKMAN TJ, HUCKER WJ, et al. 2017 ACC Expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol 2017; 69:871-98
- DOUKETIS JD. Perioperative management of patients who are receiving warfarin therapy: an evidence-based and practical approach. Blood 2011; 117:5044-9
- DOUKETIS JD, BERGER PB, DUNN AS, et al. The perioperative management of antithrombotic therapy. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133:299S-339S
- DOUKETIS JD, HEALEY JS, BRUECKMANN M, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery ot procedure. Thromb Haemost 2015; 113:625-32
- DOUKETIS JD, SPYROPOULOS AC, KAATZ S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823-33
- DOUKETIS JD, SPYROPOULOS AC, SPENCER FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, Chest 2012; 141 (2 suppl): e326S-50S
- DROUET L, BAL DIT SOLLIER C, STEINER T, et al. Measuring non-vitamin K antagonist oral anticoagulant levels: When is it appropriate and which methods should be used? Int J Stroke 2016; 11:748-58
- HOVAGUIMIAN F, KÖPPEL S, SPAHN DR. Safety of anticoagulation interruption in patients undergoing surgery or invasive procedures: A systematic review and meta-analyses of randomized trials and non-randomized studies. World J Surg 2017; doi 10.1007/s00268-017-4072-x
- KATZ J, FELDMAN MA, BASS EB, et al. Risks and benefits of anticoagulant and antiplatelet medication use before cataract surgery. Ophthalmology 2003; 110:1784-8
- MAR PL, FAMILTSEV D, EZEBOWITZ MD, et al. Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: Review of the literature and recommendations for specific populations and procedures. Int J Cardiol 2016; 202:578-85
- SIEGAL D, YUDIN J, KAATZ S, et al. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 2012; 126:1630-9
- TAFUR AJ, McBANE R, WYSOKINSKI WE, et al. Predictors of major bleeding in peri-procedural anticoagulation management. J Thromb Haemost 2012; 10:261-7
- VAN VEEN JJ, MAKRIS M. Management of perioperative anti-thrombotic therapy. Anaesthesia 2015; 70 (Suppl.1): 58-67
- WYSOKINSKI WE, McBANE RD. Periprocedural bridging management of anticoagulation. Circulation 2012; 126:486-90