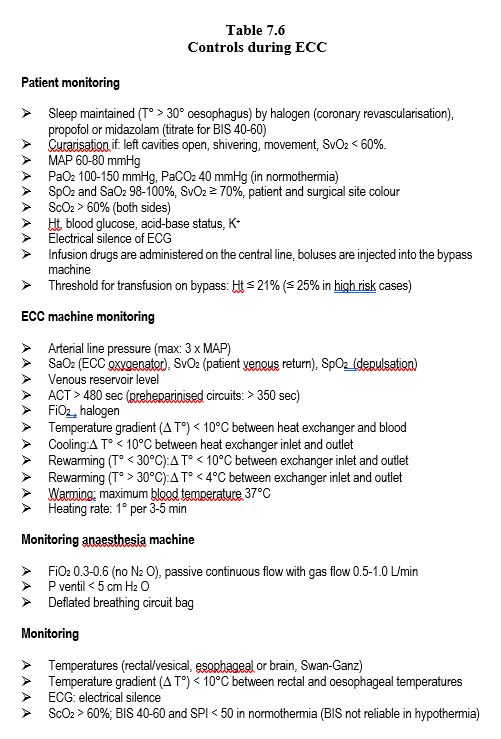
Although it is tempting to look at ECC time as a period of rest for the anaesthetist, quite the contrary he or she has a number of checks to carry out at start of (Table 7.5) and during on pump time (Table 7.6).
Like keeping on providing sleep, analgesia and muscle relaxation for the patient in a situation where the usual criteria are lost (heart and respiratory rates, blood pressure, PetCO2 , etc) [2]. In addition, bypass surgery introduces important pharmacokinetic and pharmacodynamic changes. To be rapidly effective on pump, drugs must be administered in the venous circuit of the bypass, or possibly through the central venous line, but never through a peripheral venous line where circulation is very slow, especially in hypothermia. Continuous infusions remain connected to a central venous line.
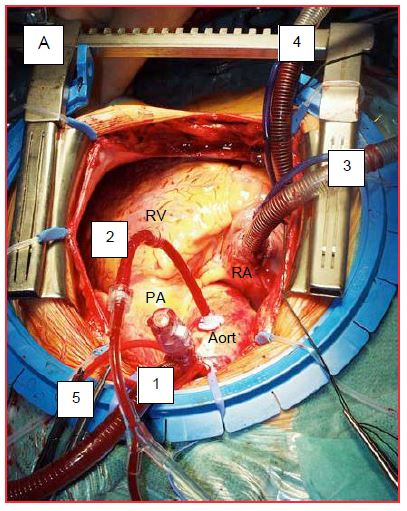
ECC can be started when the ACT has exceeded 400 seconds. With pre-heparinised circuits and mini-ECC, the desired value is lower: 300-350 seconds [1]. These values are achieved by administering intravenous (central line) or intra-auricular heparin at 300-400 IU/kg or 200-250 IU/kg, respectively. During insertion of the arterial cannula into the ascending aorta, the anaesthetist momentarily lowers the blood pressure (MAP 50 mmHg) to avoid tearing the aorta. Injecting an arterial dilator is a possibility (phentolamine 1 mg, nicardipine 0.3-1.0 mg, nitroglycerin 50-100 mcg). Once the cannula is inserted, the aortic pressure should be pulsatile in the arterial line of the ECC and identical to that of the patient. The surgeon then inserts the cardioplegia cannula into the root of the aorta. Finally, one or two venous cannulae are inserted into the right atrium to drain blood to the bypass tank (Figure 7.32). Their correct positioning is checked with TEE.
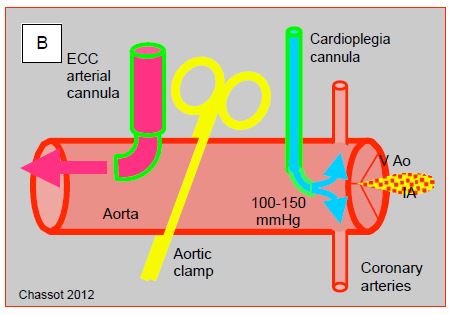
Figure 7.32: ECC cannulations. A: Cannulations in the operating field as seen by the anaesthetist. 1: arterial cannula in the ascending aorta. 2: cardioplegia cannula. 3: SVC cannula. 4: IVC cannula. 5: fixation of the aortic cannula. B: representation of the clamp and cardioplegia line; in aortic insufficiency (AI), the cardioplegia solution leaks into the LV and dilates it as it no longer ejects.
This last manoeuvre is often poorly tolerated hemodynamically by the patient: hypotension, abrupt drop in venous return, supra-junctional tachyarrhythmias. To restore blood volume, the simplest and most effective way is to infuse 100 mL bolus of the priming solution through the aortic cannula. Arrhythmias stop as soon as the pump and cardioplegia are started.
To start the bypass, the perfusionist first opens the venous cannula gradually to drain blood from the RA, then increases the arterial pump flow to the theoretical patient flow rate (2.4 L/min/m2 ). This flow rate must be reached within 2-3 minutes to avoid perfusing the organs with blood that is very diluted by the priming fluid, which causes hypotension and leads to a momentary ischaemic risk [3]. At this point, all blood is diverted into the machine, which provides full arterial flow. As soon as full flow is announced by the perfusionist, the anaesthetist interrupts mechanical ventilation. There is nothing in the literature to recommend any form of ventilation other than a passive flow of O2 /air mixture (1 L/min, FiO2 0.3-0.5) under a pressure < 5 cm H2 O [6,9].
The beginning of bypass time requires maximum attention, since incidents are then most frequent and most serious: material defects, assembly errors, disconnection, malposition of cannulas, air-lock, hypotension, hypoxaemia, aortic dissection, etc. During ECC, the most serious causes of accidents are arterial hypotension, gas embolism and oxygenator malfunction. These are followed by disconnections and inadvertent clamping [8]. Air in the arterial cannula or cardioplegia cannula is a threat of catastrophic cerebral embolism leading to massive stroke or death. Air is most often caused by inattention to the venous reservoir level or reversal between the venous and arterial lines.
Special attention is paid to the colour of venous (dark red blood) and arterial (bright red blood) cannulae. Very dark ("black") venous blood indicates central venous desaturation and low flow; arterial desaturation is typically an oxygenator problem. Saturation probes placed on the circuits allow visual observations to be objectified and quantified (see Figure 7.3). The arterial saturometry of the bypass circuit only monitors the performance of the oxygenator, not the patient; the latter is assessed by the SpO2 of the anaesthesia monitor, provided the arterial flow is pulsatile enough. The most important information is provided by the SvO2 located on the venous return circuit of the bypass machine. If the vena cava is not properly drained, stasis will set in very quickly: hepatomegaly, facial oedema, conjunctival oedema.
Transient hypotension is common at the start of the pump due to haemodilution, which suddenly lowers blood viscosity and colloidal-osmotic pressure. When present in the priming solution, aprotinin may trigger an anaphylactic reaction. Pressure is restored when hypothermia increases viscosity and causes sympathetic stimulation. Severe hypotension (MAP < 40 mmHg) should be investigated immediately as a serious incident [10].
- Dissection of the aorta: disappearance of the pressure wave on the arterial catheter while the pressure is high on the arterial cannula of the bypass circuit, and poor venous return to the machine; the ascending aorta becomes puffy and purplish. TEE display images of dissection.
- Shunt left open between the arterial and venous line.
- Evacuation of the arterial filter that remained open at too high a flow rate.
- Persistent obstruction on the arterial line.
- Inadequate flow control of the master pump.
As the patient is still normothermic and the heart is not stopped, the emergency treatment is an immediate interruption of the bypass.
The abrupt dilution of circulating substances and the sympathetic stimulation of hypothermia may wake up the patient [5]. As pulmonary output becomes minimal, only intravenous drugs administered in the heart-lung machine are effective. Depending on the type of cannulation, substances introduced through the central venous line may take up to 5 minutes to reach their target. Halogen is vaporised in the bypass oxygenator circuit.
For the anaesthetist, the transition to bypass comes along with a number of manoeuvres and controls (Table 7.5) [2].
- Mechanical ventilation is stopped, replaced by a continuous flow of O2 /air of 1 L/min (Fi O23-0.5); no N2 O [9]. This takes place as soon as the perfusionist announces that heart-lung machine is at full flow; if the operation requires it, he starts to cool down the patient’s blood. Ventilation is not interrupted in partial bypass, nor if there is a problem compromising the optimal flow of the pump.
- Removal of the Swan-Ganz catheter by 5-10 cm to prevent it from puncturing the pulmonary vessel as the lungs collapse and as it stiffens with hypothermia.
- Stop sounds and alarms on the monitor; set alarms to "ECC program".
- Maintain anaesthesia with a halogen (1-1.5 MAC) on the bypass oxygenator or an intravenous bolus (midazolam 5-10 mg) or infusion (propofol 5 mg/kg/hour) agent. Drug administration in the heart-lung machine is more precise than by the central line; do not use peripheral venous lines, which have random flow rates. Halogen is a better choice in coronary revascularisation because of the ischaemic protection offered by preconditioning (see Myocardial Protection).
- Discontinue current infusions, except for propofol if this is the chosen anaesthetic agent.
- Curarisation in case of shivering (hypothermia), opening of the left cavities, circulatory arrest or a fall in SvO2 < 60%.
- Normal pump flow is generally considered to be 2.4 L/min/m2 at 35-37°C (70 mL/kg/min) and 1.8 L/min/m2 at 28°C [11]. Cerebral blood flow is maintained constant over a flow range of 1.0 to 2.4 L/min/m2, whereas flow in the abdominal viscera decreases as soon as the flow rate falls below 2.0 L/min/m2 [4].
- Hemodynamics: MAP is maintained at approximately 50-60 mmHg. In the presence of vascular atheromatosis, diabetes, hypertension, renal insufficiency, carotid stenosis, neurological anamnesis and advanced age (> 70 years), it is preferable to maintain MAP at ≥ 70 mmHg [11].
- Cardioplegia: If there is even minimal aortic insufficiency, monitor LV size (TEE) during cardioplegia and ensure that there is no ventricular dilation. Then monitor the adequacy of cardioplegia proved by electrical silence on the ECG.
The recommended flow rate and blood pressure for bypass surgery are based on empirical evidence. The minimum tolerable mean pressure is ideally defined by the lower threshold of cerebral selfregulation, but this varies between 40 and 80 mmHg between individuals [11]. This can be judged by measuring the MAP below which cerebral O2 saturation (ScO2 ) becomes pressure-dependent (mean 66 mmHg) [7]. When necessary, MAP is raised by a vasopressor (phenylephrine, noradrenaline) or lowered by a vasodilator (phentolamine, nicardipine, nitroglycerin, momentary increase of isoflurane Fi to 2-5%). Vasoconstrictors are not given in aortic insufficiency prior to aortic clamping because of the risk of LV dilatation.
But blood pressure is not the only adjustment variable on pump. It is one of the three elements that ensure adequate O2 supply to the organs:
- Blood pressure;
- Pump flow rate;
It is vital to ensure pump flow is optimal before changing arterial resistances, as a vasoconstrictor is not the right solution if the pump flow is low. To do this, one can easily calculate the systolic arterial resistances (SAR) from the arterial resistance formula (SAR = 80 (MAP - RAP)/PF), since CO is the pump flow (PF displayed on the machine) and RAP is considered zero because it is emptied by gravity into the venous reservoir of the bypass machine. This gives us: SAR = 80 - (MAP / PF) dynes s cm-5 . Using a vasoconstrictor or vasodilator only makes sense if the SAR is abnormal. If the SvO2 is lowered to < 60%, it is more a matter of improving the pump flow and/or increasing the haematocrit.
| Starting the ECC |
| Possible start if ACT is > 400 sec
Stop ventilation and infusions when full flow is reached
Maintenance of anaesthesia with an intravenous agent (central venous or by the ECC circuit) or with a halogen (on the oxygenator)
Hypotension and risk of revival/securitisation due to haemodilution by priming fluid
Monitoring of venous return (RA cannulas and/or vena cava) and arterial cannula (risk of aortic dissection)
Sleep is maintained by continuous administration of halogen (1-1.5 MAC), propofol infusion (3-5 mg/kg/hour) or midazolam bolus/infusion (5-15 mg). Curarisation is recommended if there is shivering, opening of the left chambers, circulatory arrest or a fall in SvO2 < 60%.
MAP is maintained at 50-60 mmHg; 70-80 mmHg is preferred in diabetes, hypertension, arteriosclerosis, renal insufficiency, carotid stenosis, neurological history and advanced age.
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- ALDEA GS, DOURSOUNIAN M, O'GARA P, et al. Heparin-bound circuits with a reduced anticoagulation protocol in primary CABG: a prospective, randomized study. Ann Thorac Surg 1996; 62:410-7
- BARRY AE, CHANEY MA, LONDON MJ. Anaesthetic management during cardiopulmonary bypass: a systematic review. Anesth Analg 2015; 120:749-69
- BAUFRETON C, MOREAU X, CORBEAU JJ, et al. Standard ECC and new consumable devices. In: JANVIER G, LEHOT JJ (ed). Circulation extracorporelle: principes et pratique, 2nd edition. Paris, Arnette Groupe Liaison SA, 2004, pp 113-45
- BOSTON US, SLATER JM, ORSZULAK TA, et al. Hierarchy of regional oxygen delivery during cardiopulmonary bypass. Ann Thorac Surg 2001; 71:260-4
- BUYLAERT W, HERREGODS L, MORTIER L, et al. Cardiopulmonary bypass and the pharmacokinetics of drugs. Clin Pharmacokinet 1989; 17:10-5
- GARCIA-DELGADO M, NAVARETTE-SÀNCHEZ I, COLMENERO M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anesthesiol 2014; 27:146-52
- JOSHI B, ONO M, BROWN C, et al. Predicting the limits of cerebral selfregulation during cardioplumonary bypass. Anesth Analg 2012; 114:503-10
- KURUSZ M, WHEELDON DR. Risk containment during cardiopulmonary bypass. Semin Thorac Cardiovasc Surg 1990; 2:400-9
- LELLOUCHE F, DELORME M, BUSSIÈRES J, OUATTARA A. Perioperative ventilatory strategies in cardiac surgery. Best Pract Res Clin Anaesthesiol 2015; 29:381-95
- MEJAK BL, STAMMERS A, RAUCH E, et al. A retrospective study on perfusion incidents and safety devices. Perfusion 2000; 15:51-9
- MURPHY GS, HESSEL EA, GROOM RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009; 108:1394-417