Equipment and drugs
It may take a long time to prepare the required equipment in the operating theatre, and it is essential to have the necessary drugs ready at the appropriate dilutions for the child’s weight (see Table 14.9). The younger is the child, the more careful is the fluid administration. It is a advisable to use a system that prevents inadvertent volume overload, such as burettes and syringe pumps. Continuous rinsing of the pressure sensors adds a volume of 2-4 mL/h and flushing adds 1-2 mL/sec. These figures should be taken into account for determining crystalloid balances. For children with shunting, meticulous care must be taken to eliminate air bubbles from infusions, since these incur a risk of an R-to-L crossing and systemic embolism, especially into the brain. In our institution, a simplified calculation is used to determine the dilution of vasoactive amines in the syringe pump: to ensure that 1 mL/hour equates to 1 μg/kg/minute, the following must be prepared: 3 mg x (child's weight) ad 50 ml.
If meticulous care is not taken to ensure that the anaesthetic trolley and the child’s immediate environment is kept tidy and well-organised, the workplace quickly turns into a battlefield! All catheters, fitted with extensions and injection valves, should be fixed, labelled and placed at the top of the operating table. It must always be possible to access the respiratory circuit directly above all the tubings. Since various individuals are concerned or involved in this type of operation, the anaesthetist must retain control and centralise all informations concerning drugs administered, infusions, transfusions and crystalloid balances.
For young children with haematocrit above 45% or who are at high risk of pulmonary hypertensive crisis, the operating theatre should be heated until CPB is initiated, after which, it is normally cooled. On entering the CPB rewarming period, the theatre should be warmed up and a warming mattress activated. Blood and infusates must also be pre-warmed.
It may take a long time to prepare the required equipment in the operating theatre, and it is essential to have the necessary drugs ready at the appropriate dilutions for the child’s weight (see Table 14.9). The younger is the child, the more careful is the fluid administration. It is a advisable to use a system that prevents inadvertent volume overload, such as burettes and syringe pumps. Continuous rinsing of the pressure sensors adds a volume of 2-4 mL/h and flushing adds 1-2 mL/sec. These figures should be taken into account for determining crystalloid balances. For children with shunting, meticulous care must be taken to eliminate air bubbles from infusions, since these incur a risk of an R-to-L crossing and systemic embolism, especially into the brain. In our institution, a simplified calculation is used to determine the dilution of vasoactive amines in the syringe pump: to ensure that 1 mL/hour equates to 1 μg/kg/minute, the following must be prepared: 3 mg x (child's weight) ad 50 ml.
If meticulous care is not taken to ensure that the anaesthetic trolley and the child’s immediate environment is kept tidy and well-organised, the workplace quickly turns into a battlefield! All catheters, fitted with extensions and injection valves, should be fixed, labelled and placed at the top of the operating table. It must always be possible to access the respiratory circuit directly above all the tubings. Since various individuals are concerned or involved in this type of operation, the anaesthetist must retain control and centralise all informations concerning drugs administered, infusions, transfusions and crystalloid balances.
For young children with haematocrit above 45% or who are at high risk of pulmonary hypertensive crisis, the operating theatre should be heated until CPB is initiated, after which, it is normally cooled. On entering the CPB rewarming period, the theatre should be warmed up and a warming mattress activated. Blood and infusates must also be pre-warmed.

Standard monitoring
A number of specific items besides ECG, precordial/oesophageal stethoscopes and pressure cuffs are required for cardiac anaesthetic monitoring. It is essential to use a pulse oximeter (SpO2) since systemic oxygen saturation is the most effective criterion for determining the Qp/Qs ratio in cases of cyanotic shunting or restricted pulmonary blood flow. It very quickly alerts to any issues in terms of blood oxygenation and peripheral perfusion. In cyanotic children, who are situated on the vertical part of the oxygen-haemoglobin dissociation curve, its variations closely match variations in PaO2. Foetal Hb does not significantly alter interpretation. Young children must be fitted with two SpO2 sensors – one preductal (right arm) and the other postductal (foot). If the pulmonary artery is banded, SpO2 must remain higher than 85% for FiO2 = 0.5. New technologies enabling analysis of variations in pulse oximeter curve amplitude provide a non-invasive means of assessing the filling level and probability of infusion response. During ligation of the ductus arteriosus, SpO2 in the lower limbs is used to detect any ligation of the descending aorta, which would obviously have catastrophic consequences.
Capnometry (ETCO2) is routinely used. Its variations are greater than its absolute value, which is generally lower than that of PaCO2. The difference between PaCO2 and ETCO2 is significant for young children due to excess fresh gas from the ventilator, which dilutes exhaled air, and the site of the uptake point, which is remote from the alveolar gas. It is further increased by two phenomena [3].
- R-to-L shunting, which equates to a dead-space effect;
- Low pulmonary blood flow in the event of right-side obstruction or pulmonary artery stenosis.
This creates a negative correlation between the arterial to end-tidal CO2 gradient and Hb saturation – this gradient is particularly wide in cyanotic children [6]. If the pulmonary blood flow is manipulated (e.g. by banding), ETCO2 follows the blood flow in the PA.
Although its correlation with neurological sequelae is open to debate, cerebral O2 saturation (ScO2) (NIRS near-infrared spectroscopy) is very useful both for monitoring cerebral oxygenation and assessing peripheral circulation (see Neurological Monitoring).
Arterial catheterisation
Invasive arterial pressure measurement is necessary for any operations with CPB and for procedures through thoracotomy, with the exception of single ligation of the ductus arteriosus in premature infants. The preferred puncture sites are the femoral artery and the radial artery. In case of coarctation, aortic hypoplasia or patent ductus arteriosus, pressure and saturation must be measured upstream (right upper limb) and downstream of the lesion. Two arterial catheters are essential when performing corrections of hypoplasia or interruption of the aortic arch. In cases of coarctation, the femoral arterial catheter may be replaced by a pressure cuff on a lower limb. If the child either has or must undergo a systemic-to-pulmonary peripheral shunt (Blalock-Taussig), cannulation must not be performed on the side of the anastomosis. The puncture site is also chosen based on likely postoperative course – the radial artery is easier to immobilise in children who recover quickly, while the femoral artery is only appropriate for children who remain ventilated and immobilised. To ensure clean surgery on immunologically immature subjects, the arterial puncture is performed in surgical aseptic conditions [4].
The radial artery is cannulated with a 24-gauge or 26-gauge catheter if the child weighs less than 5 kg and 22-gauge for children weighing between 5 and 20 kg. A 2-F Seldinger catheter can also be used. The transient ischaemia rate for the first fingers is 4-6% [8]. The more peripheral a catheter is, the more it tends to show a pressure value that is lower than the actual value during and after CPB. This phenomenon is partly secondary to hypothermic vasoconstriction [10]. Radial measurements are more sensitive to this distortion than femoral measurements. The side is chosen based on the CPB cannulation and previous procedures, avoiding the side on which a Blalock shunt is present, the left radial artery in the event of coarctation, and the right radial artery in the event of aortic cannulation in the right subclavian artery.
The femoral artery is generally easier to puncture using the Seldinger technique (2-F or 3-F) and offers more reliable measurements since it is more central. The incidence of transient ischaemia of the leg is 1 to 3% in cases of prolonged cannulation [7]. However, ischaemic problems occurring along any of the arterial lines are invariably combined with episodes of low blood flow and hypercoagulability. Given the size of the catheter, the PiCCO system requires a femoral puncture in children.
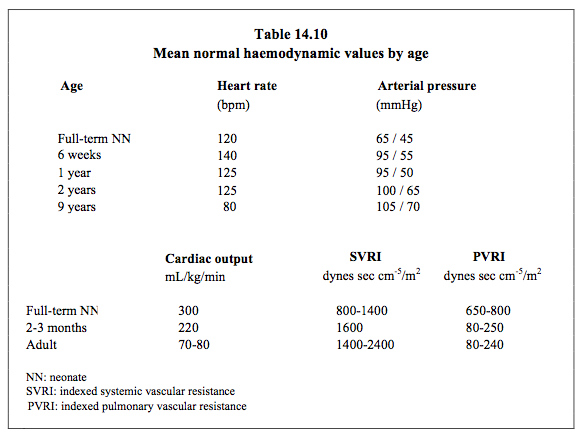
The brachial artery and axillary artery are potential back-up sites if it is difficult or impossible to access the customary radial and femoral routes. However, these are end arteries that vascularise the entire arm. The consequences of any spasms or thrombosis of these vessels are serious. One of the two umbilical arteries is accessible in the first days of life. A catheter may be inserted in it by dissecting the cord and left in place for several days. It provides adequate central arterial pressure. If percutaneous cannulation fails, there is nothing to be gained by damaging several arterial sites and wasting precious time on repeated, unsuccessful attempts. If a trained operator proceeds to a cut-down technique using microsurgical instruments, it is possible to isolate the artery and insert a catheter in it under visual inspection. Table 14.10 shows normal haemodynamic values by age.
Although its correlation with neurological sequelae is open to debate, cerebral O2 saturation (ScO2) (NIRS near-infrared spectroscopy) is very useful both for monitoring cerebral oxygenation and assessing peripheral circulation (see Neurological Monitoring).
Arterial catheterisation
Invasive arterial pressure measurement is necessary for any operations with CPB and for procedures through thoracotomy, with the exception of single ligation of the ductus arteriosus in premature infants. The preferred puncture sites are the femoral artery and the radial artery. In case of coarctation, aortic hypoplasia or patent ductus arteriosus, pressure and saturation must be measured upstream (right upper limb) and downstream of the lesion. Two arterial catheters are essential when performing corrections of hypoplasia or interruption of the aortic arch. In cases of coarctation, the femoral arterial catheter may be replaced by a pressure cuff on a lower limb. If the child either has or must undergo a systemic-to-pulmonary peripheral shunt (Blalock-Taussig), cannulation must not be performed on the side of the anastomosis. The puncture site is also chosen based on likely postoperative course – the radial artery is easier to immobilise in children who recover quickly, while the femoral artery is only appropriate for children who remain ventilated and immobilised. To ensure clean surgery on immunologically immature subjects, the arterial puncture is performed in surgical aseptic conditions [4].
The radial artery is cannulated with a 24-gauge or 26-gauge catheter if the child weighs less than 5 kg and 22-gauge for children weighing between 5 and 20 kg. A 2-F Seldinger catheter can also be used. The transient ischaemia rate for the first fingers is 4-6% [8]. The more peripheral a catheter is, the more it tends to show a pressure value that is lower than the actual value during and after CPB. This phenomenon is partly secondary to hypothermic vasoconstriction [10]. Radial measurements are more sensitive to this distortion than femoral measurements. The side is chosen based on the CPB cannulation and previous procedures, avoiding the side on which a Blalock shunt is present, the left radial artery in the event of coarctation, and the right radial artery in the event of aortic cannulation in the right subclavian artery.
The femoral artery is generally easier to puncture using the Seldinger technique (2-F or 3-F) and offers more reliable measurements since it is more central. The incidence of transient ischaemia of the leg is 1 to 3% in cases of prolonged cannulation [7]. However, ischaemic problems occurring along any of the arterial lines are invariably combined with episodes of low blood flow and hypercoagulability. Given the size of the catheter, the PiCCO system requires a femoral puncture in children.
The brachial artery and axillary artery are potential back-up sites if it is difficult or impossible to access the customary radial and femoral routes. However, these are end arteries that vascularise the entire arm. The consequences of any spasms or thrombosis of these vessels are serious. One of the two umbilical arteries is accessible in the first days of life. A catheter may be inserted in it by dissecting the cord and left in place for several days. It provides adequate central arterial pressure. If percutaneous cannulation fails, there is nothing to be gained by damaging several arterial sites and wasting precious time on repeated, unsuccessful attempts. If a trained operator proceeds to a cut-down technique using microsurgical instruments, it is possible to isolate the artery and insert a catheter in it under visual inspection. Table 14.10 shows normal haemodynamic values by age.
Central venous line
It is generally necessary to set up a central venous line (2-3 lumens) either to measure CVP, administer drugs quickly and safely during the perioperative phase, or administer fluids during the postoperative phase. Since several puncture sites are possible, consideration must be given to the inherent risks of each technique, any anatomical malformations, and the anaesthetists’ customary practices. A two or three-lumen catheter is recommended in all circumstances. Multiple punctures and several lines positioned on the same axes significantly increase the risk of superior vena cava thrombosis if haematocrit is high. Accessing the SVC is contraindicated in the Glenn procedure as thrombosis in this vein would halve the pulmonary blood flow. However, some centres use a small-diameter (4-F), single-lumen catheter to measure pulmonary pressure in it.
Although 4-F and 5-F Swan-Ganz catheters are available on the market, pulmonary artery flotation catheters are not widely used in cases of congenital heart disease due to anatomical variations that make their migration unpredictable (e.g. ASD) or impossible (pulmonary stenosis). Cardiac output measurements by thermodilution are distorted in the event of shunting. In the perioperative phase, it is possible to compensate largely for this with transesophageal or epicardial echocardiograms. Moreover, through continuous analysis of the area under systolic arterial curve (PiCCO™ system), it is possible to calculate systemic blood flow appropriately – the correlation with the calculation by thermodilution is 0.93 in children [5]. Since shunting invalidates measurement, this is particularly useful in the postoperative phase once the lesion has been corrected.
A central venous line is introduced with the same precautions as for adults (see Chapter 6 - Central Venous Line). The preferred lines are the right internal jugular vein (RIJV) and the subclavian veins. The internal jugular vein can be punctured at several sites (Figure 14.22).
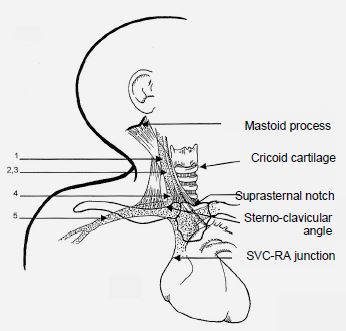
Figure 14.22: Central venous access routes to the right internal jugular vein in children. 1: Boulanger's higher access. 2,3: access at the triangle apex. 4: lower access in the sternoclavicular angle. 5: subclavian route (1 cm lateral to the centre of the clavicle in children < 10 kg, 2 cm lateral to the centre of the clavicle in children > 10 kg) [1].
An ultrasound probe is very useful for locating the vessel in young children (see Chapter 6 - Guidance by Ultrasound). Since young children have short necks and large heads, jugular cannulation is sometimes difficult due to the puncture angle. The success rate of reaching the superior vena cava is the highest from the canulation of the RIJV, although the carotid puncture rate is 10-15% in children [9]. The anatomy of the subclavian vein, when punctured in the middle third of the clavicle, is very stable. The catheter may be left very comfortably in this position for long periods. Drawbacks include catheter compression in the event of sternotomy, risk of pneumothorax, and contralateral crossing of the catheter (5-20% of punctures) [2]. The catheter insertion length (cm) may be calculated using the following formula [1]:
- Child < 100 cm: (size / 10) - 1
- Child > 100 cm: (size / 10) - 2
The catheter tip should ideally be positioned at the upper end of the pericardial reflection and parallel to the vessel wall. While the tip of the pericardium is 0.5 cm below the carina tracheae in children, it is 0.5 cm above the carina tracheae in neonates, which means that a chest X-ray is ineffective for locating the catheter [4]. The best way of monitoring the position of the catheter once it is in place is by transesophageal echocardiography: in the midesophageal bicaval view (100°), the catheter must be above the junction between the superior vena cava and the RA, which is just visible on the screen.
The femoral vein should be considered if other sites are inaccessible. It presents the same risks of thrombosis and infection as routes that are dependent on the SVC. It is situated a few millimetres more medially than the artery. In the first 2-3 days of life, the umbilical vein is easy to cannulate – the catheter passes through it via the ductus venosus up to the inferior vena cava and its tip appears above the diaphragm on a chest X-ray (see Figure 14.3) [4].
Transthoracic catheters
Complex pathologies affecting young children create significant asymmetry between the various cardiac chambers and major postoperative haemodynamic lability. As a result of this situation, more monitoring points are required than for older children or adults. If continuous monitoring of PAP or PAWP is necessary in the postoperative phase and it is not possible to insert a Swan-Ganz catheter due to the child’s anatomy or size, it is possible to surgically fit a transthoracic catheter in the PA and/or LA after CPB. A 20 to 22-gauge single-lumen central catheter measuring 10-15 cm is used for this purpose. A purse string around the puncture site limits blood loss when the catheter is removed. Two risks are associated with this type of cannulation.
The femoral vein should be considered if other sites are inaccessible. It presents the same risks of thrombosis and infection as routes that are dependent on the SVC. It is situated a few millimetres more medially than the artery. In the first 2-3 days of life, the umbilical vein is easy to cannulate – the catheter passes through it via the ductus venosus up to the inferior vena cava and its tip appears above the diaphragm on a chest X-ray (see Figure 14.3) [4].
Transthoracic catheters
Complex pathologies affecting young children create significant asymmetry between the various cardiac chambers and major postoperative haemodynamic lability. As a result of this situation, more monitoring points are required than for older children or adults. If continuous monitoring of PAP or PAWP is necessary in the postoperative phase and it is not possible to insert a Swan-Ganz catheter due to the child’s anatomy or size, it is possible to surgically fit a transthoracic catheter in the PA and/or LA after CPB. A 20 to 22-gauge single-lumen central catheter measuring 10-15 cm is used for this purpose. A purse string around the puncture site limits blood loss when the catheter is removed. Two risks are associated with this type of cannulation.
- Accidental administration of substances and/or air directly into the LA – the catheter must be clearly labelled to prevent any risk of confusion with a central venous line.
- The ablation of the catheter on the 2-3rd day may result in tamponade.
| Equipment and monitoring of children for cardiac surgery |
|
ECG, SpO2 (upper + lower limb), ETCO2, cerebral oxygen saturation (ScO2), TEE (depending on tolerability if < 3 kg), T°C (oesophageal/tympanic + rectal), urinary catheter, nasogastric tube, peripheral venous lines, arterial catheter (possibly radial + femoral), 2-3 lumen central line, possibly PiCCO for the post-CPB/postoperative period.
|
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
References
- ANDROPOULOS DA, BENT ST, SKJONSBY B, et al. The optimal length of insertion of central venous catheters for pediatric patients. Anesth Analg 2001; 93: 883-6
- ANDROPOULOS DA, STAYER SA, BENT ST. A controlled study of transesophageal echocardiography to guide central venous catheter placement in congenital heart surgery patients. Anesth Analg 1999; 89:65-70
- BEUSCH M, LENZ G, KOTTLER B. Arterial to end-tidal CO2 gradients in infants and children with cyanotic and acyanotic congenital heart disease during cardiac surgery. J Cardiothorac Vasc Anesth 1990; 4:S128
- DETAILLE T, PIROTTE T, VEYCKERMANS F. Vascular access in the neonate. Best Pract Res Clin Anesthesiol 2010; 24:403- 18
- FAKLER U, PAULI C, BAILING G, et al. Cardiac index monitoring by pulse contour analysis and thermodilution after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2007 ; 133 :224-8
- FLETCHER R. The relationship between the arterial to end-tidal PCO2 differences and hemoglobin saturation in patients with congenital heart disease. Anesthesiology 1991, 75:210-6
- GRAVES PW, DAVIS AL, MAGGI JC, et al. Femoral artery cannulation for monitoring in critically ill children: Prospective study. Crit Care Med 1990; 18:1363-6
- HACK WWM, VOS A, OKKEN A. Incidence of forearm and hand ischaemia related to radial artery cannulation in newborn infants. Intensive Care Med 1990,;16:50-3
- MALLINSON C, BENNETT J, HODGSON P, et al. Position of the internal jugular vein in children: A study of the anatomy using ultrasonography. Paediatr Anaesth 1999; 9:111-4
- RICH GF, LUBANSKI RE, MCLAUGHLIN TM. Differences between aortic and radial artery pressure associated with cardiopulmonary bypass. Anesthesiology 1992; 77:63-6
