- Determine the mechanism of MI;
- Quantify the degree of insufficiency by combining a number of elements.
As a reminder, mitral insufficiency is divided into two categories: primary MI (structural damage to the leaflets, annulus and subvalvular apparatus) and secondary MI (normal valve but left ventricular damage/dysfunction). In addition, MI is usually classified into three types according to the displacement of the free edge of the leaflets from the plane of the annulus (Carpentier classification) (see Figure 11.67) [4].
- Type I: normal leaflet motion and structure;
- Type II: excessive leaflet movement (tilting in the LA);
- Type III: restrictive leaflet movement:
- IIIa: systolic-diastolic restriction (rigid, poorly mobile leaflets);
- IIIb: systolic restriction only (trapped in LV during systole).
Types II and IIIa are structural primary MIs, whereas types I and IIIb are functional secondary MIs.
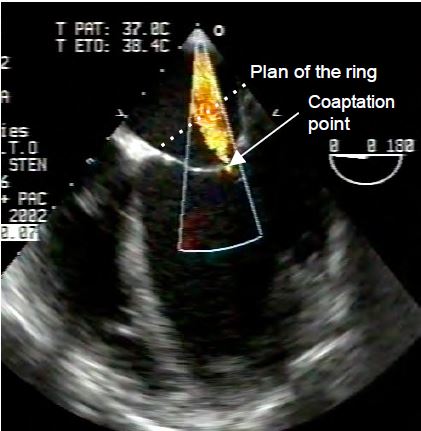
The orientation of the mitral valve is about 60° from the orthogonal planes of the body. Its orthogonal planes are therefore the bicommissural view at 40-60°, which intersects the two commissures, and the long-axis view at 120-140°, which is perpendicular to it (see Figure 11.21). The crescent-shaped posterior leaflet is anatomically divided into 3 festoons: P1 anteriorly, P2 medially and P3 posteriorly. The more quadrangular anterior leaflet is similarly divided into A1, A2 and A3, although this does not correspond to any anatomical entity. As echocardiographic landmarks, the aortic valve is adjacent to the base of the anterior leaflet and the left atrial appendage is opposite the anterior commissure. The coaptation point of the valve is below the plane of the mitral annulus; given the saddle shape of the mitral annulus, the position of the coaptation point should be assessed in the 120-140° long axis view and not in the 0° 4-cavity view (see Figure 11.3).
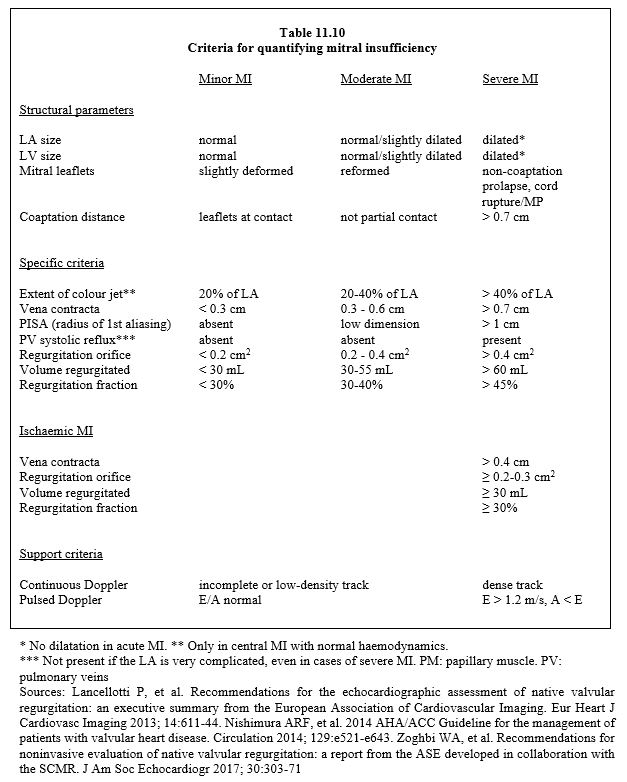
MI is usually graded into three degrees (mild, moderate or severe) according to different criteria based on two-dimensional imaging, spectral Doppler or colour Doppler and calculated using the Bernouilli or continuity equation (Table 11.10) (see Chapter 26, Mitral insufficiency for more details). Any echocardiographic index is inherently limited in scope and should never be used as the sole basis for an indication for surgery. This is the result of the integration of all the parameters assessed.
2D examination
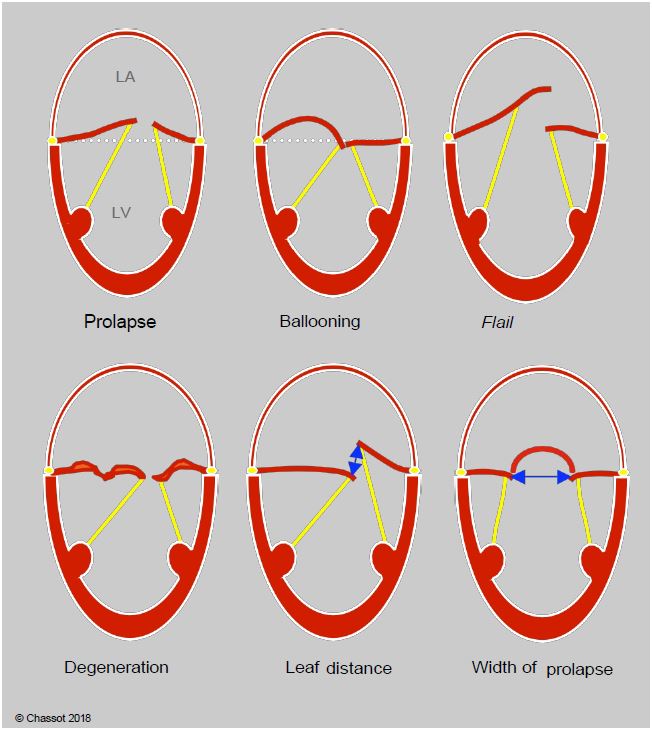
Degenerative diseases are the most common cause of primary MI. They present with different types of lesions (Figure 11.73A) [24].
- Morphological abnormality: the leaflets are thickened, redundant, bumpy; their length is excessive (myxoid degeneration, Barlow's disease).
- Ballooning: the excess valvular tissue gives the appearance of a bulging dome in the LA in systole, but the coaptation point is maintained below the plane of the mitral annulus.
- Flail leaflet: the eversion of the end of a leaflet is usually associated with the stretching or breaking of a chord.
- Prolapse: The point of coaptation is > 2 mm posterior to the plane of the mitral annulus in the LA (measured in the long-axis view at 120-140°). The 2D image can be used to measure the distance between the 2 leaflets in systole and the width of the dome created by the prolapse.
- ARF: the commissures are fused, the leaflets are fibrotic and rigid; MI is due to their lack of mobility, making them restrictive in systole and diastole; it is often accompanied by a stenosis component.
Figure 11.73A: Differentiation of degenerative lesions. In prolapse, the coaptation point is located ≥ 2 mm posterior to the plane of the mitral annulus, within the LA. In ballooning; the coaptation point is normally located, but excess valve tissue causes systolic bulging of the leaflet body. In tilting, all or part of a leaflet is tilted into the LA, most often with rupture of the chord(s). Degeneration is characterised by reshaped, thickened, bumpy leaflets with excess tissue (Barlow). The 2D image can be used to measure the distance between the leaflets in systole, which represents the extent of the MI, and the width of the prolapse, in this case P2 in the bicommissural view (60°).
In secondary MI, the leaflets are normal and the pathology is in the left ventricle, which has undergone significant remodelling. There are two main scenarios: myocardial ischaemia with abnormal segmental kinetics and ventricular dilatation. Massive dilatation of the LA leading to mitral annular distension is a third possibility. The echocardiographic picture is characteristic (Figure 11.73B).
- Dilatation of the annulus (diameter > 35 mm) prevents normal coaptation and leaves a persistent orifice in systole.
- The leaflets are of normal morphology but do not coapitate in systole; they are held below the plane of the mitral annulus by excessive chordal traction due to ventricular dilatation (symmetric restrictive MI) or akinesia of one wall (asymmetric restrictive MI).
Figure 11.73B: Restrictive mitral regurgitation due to LV dilatation and dysfunction. The point of coaptation of the mitral leaflets in systole is maintained below the plane of the mitral annulus, causing a central leak into the LA.
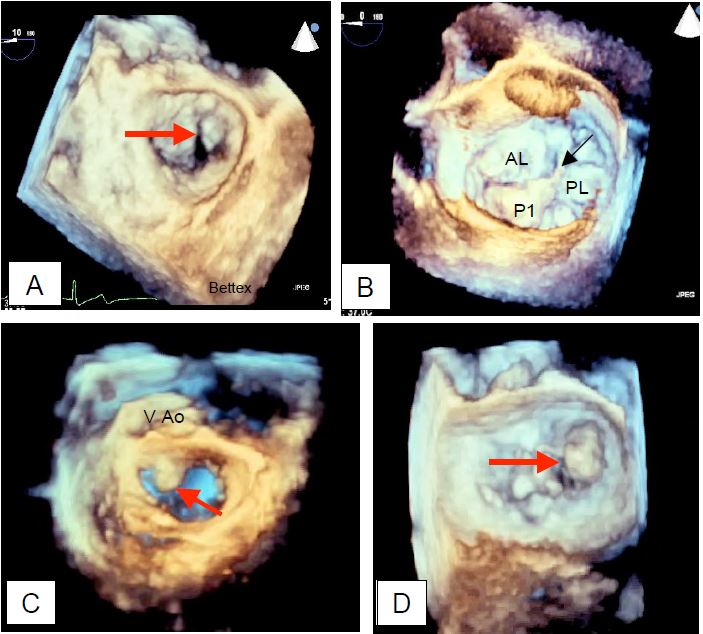
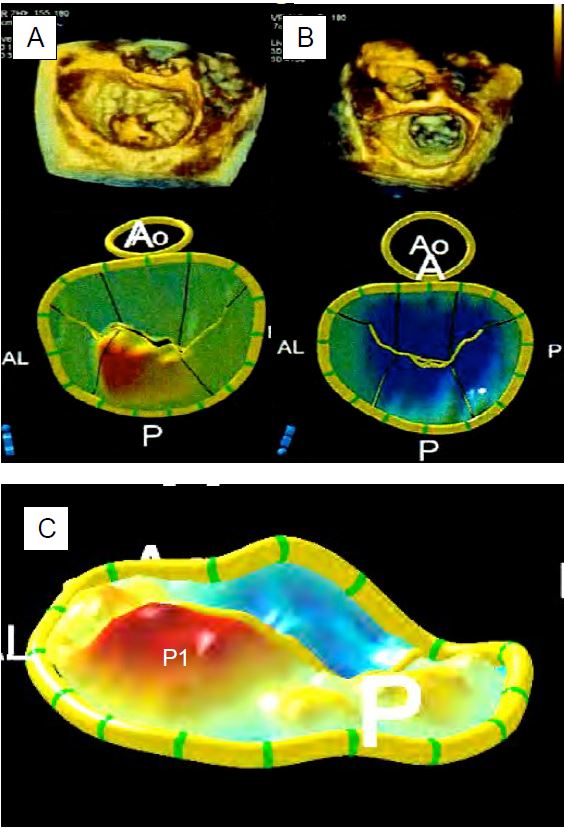
The tilting of a leaflet in the LA, non-coaptation leaving a large orifice in systole and rupture of the chords or papillary muscle are pathognomonic of a severe MI. 3D reconstruction, including an "en-face" view of the mitral valve from the LA as seen by the surgeon, allows the diagnosis to be refined and the structures to be measured with greater precision; in general, 2D views tend to underestimate the extent of prolapse compared with 3D measurements because the plane of section is relatively arbitrary compared with the maximum dimensions of the lesions (Figure 11.74) [20].
Colour Doppler MI jet
The extent of the colour jet is an excellent way of detecting an MI and getting an idea of its size, but it is by no means a way of quantifying the size of the regurgitation (Figure 11.75). The flow of the MI seen on the screen is a mapping of velocities but in no way represents the volume regurgitated. The 4-degree classification based solely on the size of the swirling jet in the LA is no longer recommended because the size of the jet depends on the colour and grey scale settings, the angle of the Doppler beam, the velocity of the red blood cells, the ventricular function, the haemodynamic conditions and the compliance of the LA and is inversely proportional to the size of the regurgitant orifice. While the combination of a good LV and a small orifice produces a high velocity jet that is very bright on the screen, the combination of a defective LV and a large orifice produces a low velocity jet with little swirl. The effective regurgitant volume is overestimated in the first case and underestimated in the second [5]. On the other hand, a high-velocity central jet tends to increase due to the suction effect on blood already in the LA, whereas an eccentric jet slows down at the wall and decreases in intensity. An MI should be assessed over a period of regular rhythm and normal heart rate, avoiding post-extrasystolic amplification; in the case of AF, multiple measurements are averaged over a period of 2-4 cycles at a rate of approximately 60-80 beats/min. In general, a central jet in the LA indicates the presence of a functional MI, whereas an oblique and eccentric jet indicates an organic MI associated with leaflet pathology.
Figure 11.74: 3D imaging of MI seen from the LA. A: Cleft of the anterior leaflet; this pathology can be very difficult to demonstrate in 2D. B: Mitral prolapse of P1; with a torn chord at the end of the prolapse (arrow). C: Prolapse of the anterior leaflet (arrow) observed in protosystole. D: Prolapse of the posterior commissure.
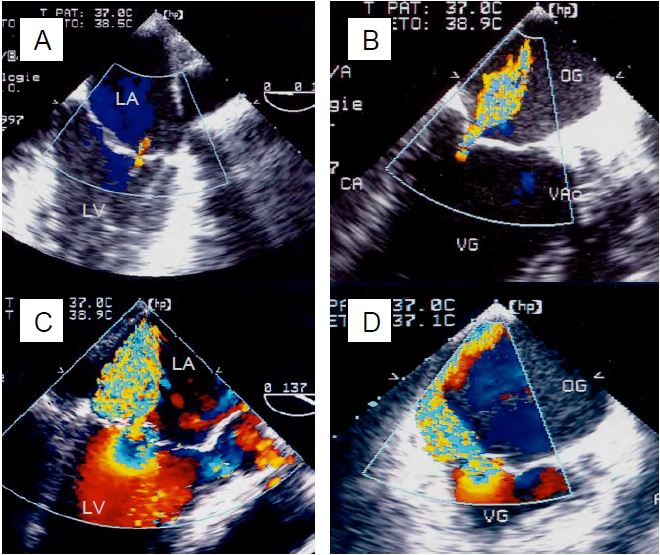
Figure 11.75: Colour Doppler TEE images of mitral regurgitation. A: Mild MI. B: Moderate MI. C: Severe central MI. D: Severe eccentric MI. The extent of the colour jet allows a good assessment of the extent of the MI, but does not allow true quantification.
Vena contracta
As it passes through a narrowed passage, the flow accelerates in a laminar fashion and contracts. The maximum contraction occurs a few millimetres beyond the orifice, at a point known in hydrodynamics as the vena contracta (Figure 11.76A). As the blood flow is still laminar, the cross-sectional area of this zone faithfully reproduces the shape of the orifice, but is slightly smaller than that of the anatomical orifice (Geometrical Orifice Area). Measurement of the diameter of the vena contracta is an excellent technique for assessing the size of the effective regurgitant orifice area (EROA) (see Doppler echocardiography). 2-3 measurements taken in two orthogonal planes should be averaged to obtain a consistent measurement. The diameter values are: < 3 mm for mild MI, 3-6 mm for moderate MI and ≥ 7 mm for severe primary MI [24,46]. The measurement is valid for both central and eccentric jets.
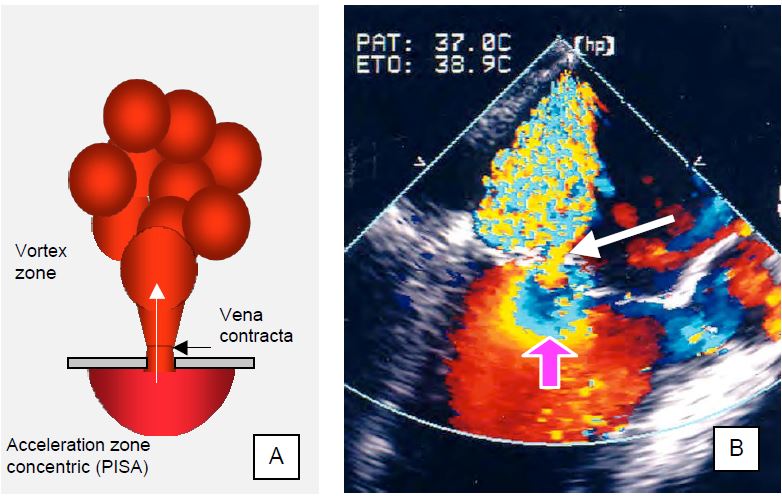
- Mild MI: < 0.2 cm2 (Vreg < 30 mL)
- Moderate MI: 0.2-0.4 cm2 (Vreg 30-55 mL)
- Severe MI: > 0.4 cm2 (Vreg > 60 mL)
Despite its performance, the calculation of SOR and Vreg derived from PISA depends on the one-dimensional measurement of a three-dimensional element performed at a precise moment in systole, whereas the phenomenon varies during ejection (see below: primary vs secondary MI). In this way, an image obtained at a precise moment is extrapolated to the whole systole, which can lead to a significant overestimation of the MI; the TM colour mode makes it possible to see the variations in intensity during ejection and to correct this error, at least partially. As with any colour Doppler, the measurement is influenced by the colour scale (in this case 20-40 cm/s), the grey scale (in this case 50%) and the angle between the flow and the analysis axis [8]. Other limitations must also be taken into account: low accuracy in eccentric MI, difficulty in defining the position of the mitral orifice in the middle of the colour flow, assumed circular shape of the orifice, geometry of the PISA modified by adjacent structures, assumed synchronisation between Vmax of the MI and Vmax of the PISA [5,16]. In addition to automatic calculations, new echo platforms are beginning to incorporate shape recognition algorithms to limit the error and variability of measurements.
Spectral Doppler
Pulsed Doppler examination of anterograde mitral flow, with the sample volume positioned between the leaflet ends where flow velocity is greatest, shows an E flow with a velocity of ≥ 1.5 m/s. This acceleration is due to the large volume passing through the mitral valve at the beginning of diastole, as the normal filling volume of a cardiac cycle is increased by the volume regurgitated during the previous systole. The ratio of integral velocity through the mitral valve to that through the aortic valve (VTIMV/VTIVAo ) is > 1.4 in severe MI. These observations essentially apply to primary MI; in secondary MI it becomes difficult to distinguish the increase in E flow Vmax due to mitral regurgitation from that due to restrictive diastolic dysfunction [5].
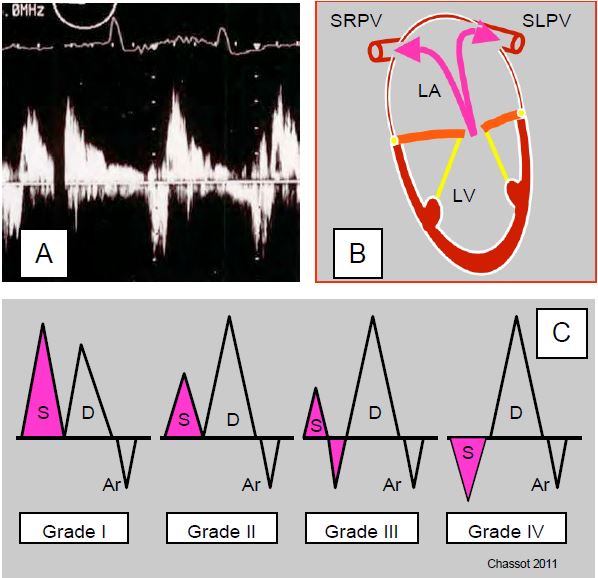
Figure 26.35: Systolic reflux in the pulmonary veins. A: Pulsed Doppler spectral image in the left upper pulmonary vein. B: The right veins are at a smaller angle to the axis of a symmetric central MI than the left veins. C: The systolic component of pulmonary venous flow is not modified by a trace of MI, but is decreased in moderate MI, partially reversed in moderate to severe MI, and reversed in severe MI; this progressive reflux is absent when the LA is dilated and very compliant.
By placing the sample volume 1-2 cm inside a pulmonary vein (PV), we observe the continuous biphasic flow typical of the large central veins, accompanied by a very slight reflux of short duration at the time of atrial contraction, due to the absence of a valve between the LA and the PVs. The systolic (S) component of the anterograde flow depends on the pressure in the LA because the mitral valve is closed at this time. If the mitral valve is leaky, the S component is attenuated; in severe MI it is reversed. This massive systolic reflux in the PVs is an excellent criterion for severe MI when it is present, but its absence by no means rules out severe MI. In fact, this reflux depends on the compliance of the LA (a very dilated atrium absorbs the regurgitant flow) and the direction of the jet (an eccentric jet can reflux into the PVs on one side only). To be significant, systolic reflux in the PVs must be observed on both sides (see Figure 26.35).
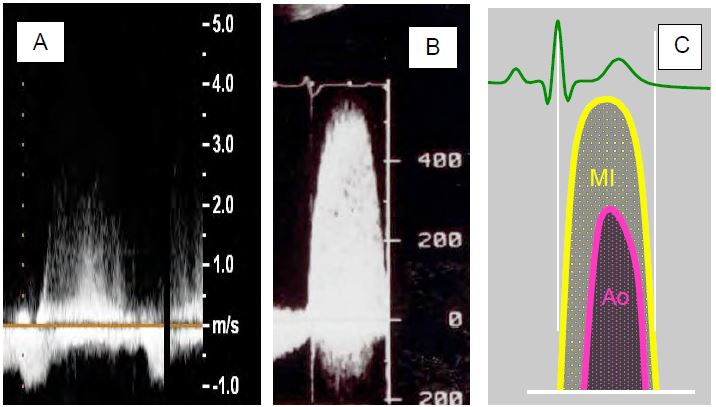
Continuous Doppler examination of MI flow will show a Vmax of 4-6 m/s and a denser trace the larger the MI. Both MI and aortic stenosis produce accelerated systolic flows away from the apex. On transthoracic and transesophageal examination, these two flows can be confused, but their morphology distinguishes them. The MI flow is rounded, begins during the isovolumetric contraction phase and ends during the isovolumetric relaxation phase, whereas the more aortic flow has a more triangular shape and a duration reduced to the ejection phase (Figure 11.77).
Figure 11.77: Spectral Doppler flow in MI (continuous Doppler). A: Doppler flow of a moderate MI; the trace is not very dense and the envelope is uncertain. B: Spectral Doppler flow; Vmax is 5-6 m/s, the trace is compact and the envelope is well defined. C: Mitral flow begins as soon as PLV exceeds 5-8 mmHg, well before the aortic valve opens and systemic flow begins, so there is no true isovolumetric phase. Aortic flow is more triangular and lasts only during the ejection phase.
Quantifying MI
In summary, severe mitral regurgitation is characterised by the following criteria (see Table 11.10). [2,14,23,24,32].
- LV dilatation (short-axis end-diastolic diameter > 4 cm/m2 ) (absent in acute MI);
- LA dilatation (diameter > 5 cm) (absent in acute MI);
- Non-coaptation of the leaflets or tilting of a leaflet in systole;
- Regurgitant jet traversing the entire LA (colour Doppler);
Video: Severe central mitral insufficiency; the MI jet is wide, occupying a large part of the LA and reaching its posterior wall.
- Holosystolic regurgitation (TM colour mode);
- Diameter of regurgitant jet at its origin (vena contracta) ≥ 0.7 cm;
- Large area of intraventricular flow convergence (PISA): radius of 1st aliasing > 1.0 cm (colour scale 40 cm/s);
Video: severe eccentric mitral insufficiency due to prolapse of the posterior leaflet; the PISA is highly developed.
- Regurgitant orifice > 0.4 cm2;
- Regurgitant volume ≥ 60 ml (this value correlates best with clinical prognosis);
- Regurgitant fraction > 50%;
- Systolic regurgitation in the pulmonary veins (inconsistent);
- PAPsyst > 60 mmHg on exertion.
Severity criteria are more restrictive for ischaemic failure, reflecting severe ventricular pathology. Secondary ischaemic or dilatative MI is generally considered severe if the following criteria are met [16]
- Vena contracta diameter > 0.4 cm;
- Regurgitant orifice > 0.2 cm2;
- Regurgitant volume > 30 mL.
The diagnosis and quantification of MI is based on a series of converging data, rather than on a single element that may be misleading. The most reliable criteria are those that are least dependent on haemodynamic conditions, such as 2D and 3D imaging, vena contracta diameter, the size of the acceleration zone (PISA) and the surface area of the regurgitant orifice [15,39]. In acute failure, the morphology of the LA and LV is not altered; remodelling is absent, unless the acute MI is a progressive accident in the course of a chronic lesion.
The TEE performed in the operating theatre is often inconsistent with the transthoracic examination previously performed by the cardiologist. In fact, general anaesthesia and positive pressure ventilation significantly modify LV preload and afterload to the extent that the estimation of MI severity decreases by 25-30% under GA [17]. The extent of the colour jet and the diameter of the vena contracta are significantly reduced after induction, whereas SRO and Vreg are little changed. Restoration of afterload by a vasoconstrictor (phenylephrine) increases Vreg without significantly changing SRO [37]. We can therefore see that GA reduces MI and that its measurement tends to underestimate it in 50% of patients, but that artificial restoration of haemodynamics leads to an overestimation in 20% of patients. In mitral surgery, the aim of intraoperative TEE is not so much to quantify MI, which has a high margin of error, but to determine the anatomical basis of the disease, to define the chances of success of a procedure and to guide the surgeon in the choice of technique.
LV Function
For the LV, MI is a godsend ! It has sufficient preload at normal pressure to position itself ideally on its Starling curve, and its afterload is kept low by the pressure valve provided by the MI. The mitral valve leaks as soon as the intraventricular pressure exceeds the PLA, i.e. at the beginning of the isovolumetric contraction phase. Under these conditions, its ejection fraction is excellent, even if part of the stroke volume is ejected posteriorly. Significant functional impairment is required for EF to become abnormal, making it a less sensitive indicator of systolic performance in MI. The LV only begins to suffer as it dilates and becomes increasingly spherical. In these circumstances, ventricular performance is best assessed by the thickness and size of the ventricle (diameter D and short axis area S) in telesystole [3].
- Normal maximum LV diameter (Dts): 4 cm, or 2.5 cm/m2;
- Maximum normal short axis (ETO) surface area (Sts): 6.5 cm2/m2 .
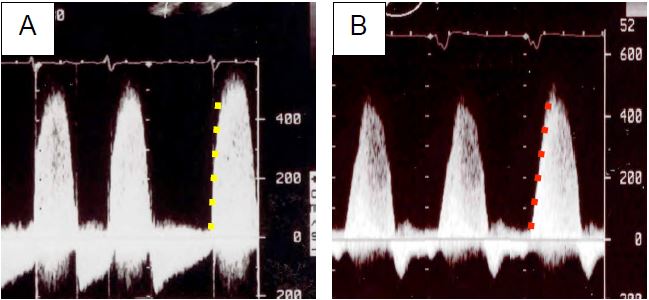
The presence of an MI makes it possible to measure intraventricular dP/dt, since the valve leaks into the LA as soon as the contraction begins. The acceleration of the MI flow (upward slope of the spectral flow) then reflects the dP/dt of the ventricle. TEE measures the time elapsed between the moment when the velocity of the MI is 1 m/s and the moment when it is 3 m/s. These velocities correspond to a pressure gradient between the LV and LA of 4 and 36 mmHg, respectively (Bernouilli equation: ΔP = 4 V2 ); the normal value of dP/dt is 1,200 to 2,000 mmHg/s (see Figure 26.19) [43]. Put simply, the function is normal if the acceleration from 1 to 3 m/s takes less than 27 msec. Any deceleration indicates a loss of LV power. This index has the advantage of being independent of afterload as the aortic valve is not yet open during the measurement.
Figure 26.19: Spectral Doppler flow image of mitral regurgitation. The dense, compact pattern indicates severe MI. A: Normal LV function, Vmax > 5 m/s and very steep ascending gradient. B: Reduced LV function, lower Vmax and smaller gradient.
3D Exam
Three-dimensional mode significantly fills the gaps left by 2D mode for examination of mitral structures and haemodynamic calculations. Real-time 3D modes are invaluable for visualising the mitral apparatus in motion, including the "en-face" image of the valve, where it is viewed from the LA (operative view); the image is positioned so that the aortic valve is at 12 o'clock and the left atrial appendage is at 10 o'clock [25]. From this mid-esophageal retrocardiac position, the TEE probe can be advanced to the transgastric position where it provides an excellent image of the subvalvular apparatus. Real-time 3D (X-plane, live 3D and zoom) is particularly useful for catheter guidance during percutaneous procedures [35]. Full-volume 3D imaging allows cropping in any plane and highlighting of specific elements (leaflets, annulus) by removing adjacent tissue. Using appropriate algorithms, we can directly measure the regurgitant orifice surface area, leaflet surface area, prolapse volume, tent volume, annulus non-planarity, papillary muscle location, volumetric quantification of the MI jet, etc. Volumetric assessment of the LV, independent of any geometric approximation, allows reliable calculation of stroke volume and ejection fraction, but is dependent on the acuity of automatic detection of the ventricular cavity contours [7,19,25].
Superimposing the colour jet on a full-volume 3D image defined by a fixed grey scale allows an acquisition frequency of 30-40 Hz, giving approximately 12 images per systole; in off-line reconstruction, it is possible to pass the slice plane through the narrowest part of the vena contracta and measure its surface, regardless of its shape, provided it is perpendicular to the flow [6]. A new image field optimisation technology allows automatic calculation of the surface area of the PISA and SRO from a 3D examination with real-time colour Doppler; correlation with manual calculations is excellent and variability is greatly reduced because the system does not assume that the PISA is hemispherical, but calculates the actual surface area regardless of its shape [40]. Semi-automated parametric modelling programs can extract sutures, such as the mitral annulus and leaflets, from full-volume data and then make a series of measurements on them (Figure 11.78) [35,40].
- Anteroposterior diameter of ring: normal < 1.6 cm/m2 (Barlow: > 2.5 cm/m2);
- Circumference of annulus: normal < 5 cm2 /m2 (Barlow: > 7.5 cm2 /m2 );
- Anterior leaflet surface area: normal < 2.75 cm2 /m2 (Barlow: > 4.75 cm2 /m2 );
- Intercommissural diameter: normal < 1.8 cm/m2 with no change during systole (Barlow > 1.8 cm/m2 with enlargement in telesystole)
- Mitral annulus elevation (saddle height): normal 1 cm (Barlow < 0.8 cm);
- Balloon height: fibroelastic degeneration < 1 cm, Barlow > 1 cm;
- Balloon volume: fibroelastic degeneration < 1.15 cm3, Barlow > 1.15 cm3;
- Tent volume (severe secondary MI: > 1.5 cm).3
Figure 11.78: 3D reconstructions of the mitral valve and annulus; full volume images at top and parametric images of the mitral annulus and leaflets at bottom. A: P2 prolapse (type II MI). B: Restrictive MI (type IIIb). The regurgitant orifice is not circular as assumed by conventional calculations, but clearly slit-shaped. C: Magnified image of P1 prolapse. The blue parts are located below the level of the ring, the red parts are located above the level of the ring [from: Poelaert JI, Bouchez S. Perioperative echocardiographic assessment of mitral valve regurgitation: a comprehensive review. Eur J Cardio-Thor Surg 2016; 50:801-12].
Primary vs. secondary MI
The extent of secondary MI is much more dynamic and variable than that of primary MI because the former is related to ventricular function, whereas the latter is determined by structural damage to the leaflets. The regurgitant orifice area of secondary MI increases significantly on exercise testing but decreases significantly under anaesthesia due to the reduction in preload, afterload and sympathetic tone. Inotropic agents such as dobutamine also contribute to its reduction by reducing LV telesystolic volume, unless they induce acute myocardial ischaemia [22]. The lack of effect of dobutamine on MI suggests a lack of contractile reserve, which is a predictor of postoperative ventricular dysfunction. An increase in preload (filling, Trendelenburg) or afterload (vasopressor) forces LV dilatation and increases secondary regurgitation. On the other hand, MI due to prolapse or dynamic obstruction of the LVOT is reduced under these conditions, because LV dilatation exerts a tension on the chords, bringing the prolapse back towards the coaptation plane in the first case, or counteracting the SAM effect in the second case [34].
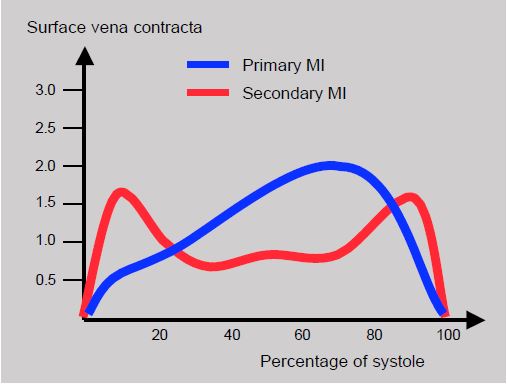
The regurgitant volume, an element directly related to prognosis, depends on the duration of opening of the regurgitant orifice during systole. The surface area of this orifice is a dynamic parameter that varies during ejection. The area of the vena contracta (SVC), which is modelled on the area of the regurgitant orifice in systole, shows a telesystolic peak in primary MI (110% increase). In secondary MI, the variation in SVC is biphasic, with an increase in proto- and telesystole (27%), but a decrease in mesosystole [6]. When the TM mode is applied to the colour flow of the MI, the same phenomenon is observed: in primary MI, the peak size of the PISA occurs in the second half of systole, whereas in secondary MI there is a proto-systolic peak and a second telesystolic peak (Figure 11.79). The reason for this peculiar configuration of secondary MI is the slight narrowing of the mitral leakage surface when intraventricular pressure, which tends to close the mitral valve, is at its maximum in mesosystole. In primary MI, the increase in intraventricular pressure during systole tends to increase the prolapse of the diseased leaflet. This phenomenon demonstrates the irrelevance of measurements taken on a still image chosen at random during systole and the difficulty of comparing primary and secondary MI using the same criteria.
Figure 11.79: Evolution of MI during systole described by that of the vena contracta surface. Red: functional MI; biphasic temporal distribution with a mesosystolic decrease. Blue: structural MI (Barlow's disease); the area of the vena contracta increases progressively to peak in telesystole [6].
Secondary MI
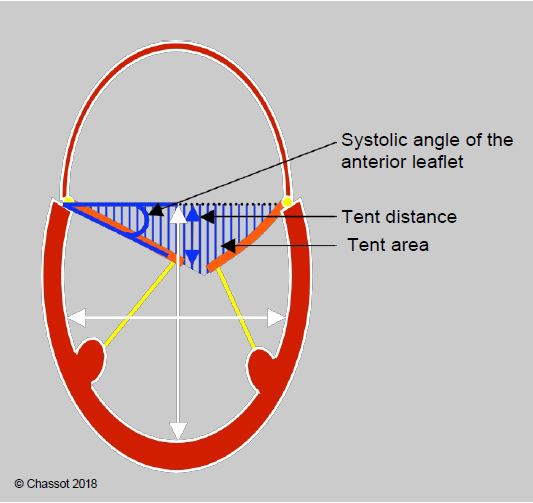
The prevalence of secondary MI is 28% in patients with myocardial ischaemia and 50% in patients with left ventricular failure [42,44]. The discovery of a significant MI on pre-ECC TEE is therefore a common occurrence for the anaesthetist echocardiographer. This failure is type IIIb, symmetrical in the case of LV dilatation and asymmetrical in the case of focal ischaemia. The echocardiographic feature of secondary MI is that one or both mitral leaflets remain below the plane of the mitral annulus in systole due to excessive traction exerted by the chords anchored to a locally or globally dilated ventricular wall. The coaptation point is located at a certain intraventricular distance from the plane of the annulus. The triangle formed in systole by the plane of the annulus and the two leaflets resembles a tent (upside down in TEE but right side up in TTE) and determines some parameters that allow quantification of the severity of the degree of mitral deformation associated with ventricular pathology (Figure 11.80) (see Secondary ischaemic MI) [33,38].
- Ring diameter (> 3.4 cm);
- Distance between the coaptation point and the plane of the ring: tenting distance ≥ 1.1 cm;
- Area between the plane of the annulus and the mitral leaflets: tenting area ≥ 2.5 cm2;
- Volume between the plane of the annulus and the mitral leaflets (3D): tenting volume ≥ 3.0 cm3 ;
- Angle of the anterior leaflet to the annulus plane (> 20°);
- Angle of the posterior leaflet to the annulus (> 30°);
- "Seagull sign" deformation of the anterior leaflet due to traction on the secondary cords implanted in the body of the leaflet; this occurs when a papillary muscle is displaced outwards due to ischaemia of the underlying wall (posterior or anterolateral) (Figure 11.65B).
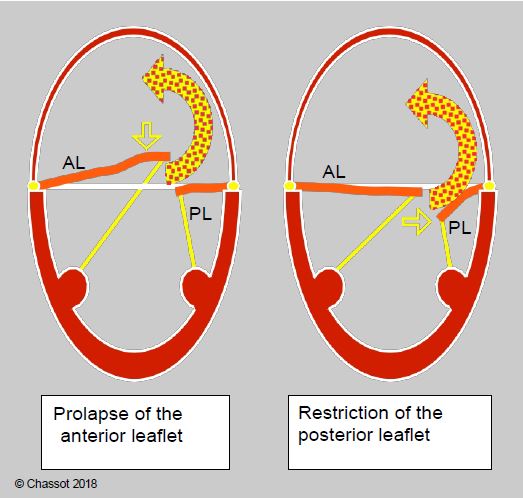
- Pseudo-prolapse: excessive traction on one leaflet while the other remains in place produces an eccentric jet of MRI which may be confused with that of prolapse of the opposite leaflet (Figure 11.81).
Figure 11.80: Restrictive MI (type IIIb) in a dilated LV. The degree of restriction is defined by the distance from the point of coaptation to the plane of the mitral annulus (tenting distance), the triangular area between the leaflets and the plane of the annulus (tenting area) and the angle of closure of the anterior leaflet measured between the plane of the annulus and the tip of the leaflet [35]. The ratio of LV long axis to short axis diameter defines the degree of sphericity (normal long axis to short axis ratio: > 1.5).
Figure 11.81: Eccentric mitral regurgitation directed towards the lateral-posterior wall of the LA; it may be due to prolapse of the anterior leaflet (AL) or regurgitation of the posterior leaflet (PL). In the former, the anterior leaflet is tilted behind the plane of the mitral annulus (true prolapse), whereas in the latter, the posterior leaflet remains below the plane of the annulus (false prolapse). In both cases the jet has the same direction.
These measurements are taken from a mid-esophageal 4-cavity 0° and long-axis 120° telesystole view. Secondary MI is considered severe when the regurgitant orifice area (calculated by PISA or vena contracta) is > 0.2 cm2 and the regurgitant volume is > 30 mL. In addition to the problem of the crescent-shaped, non-circular shape of the regurgitant orifice, there is the problem of ischaemic or functional damage to the LV, which can be decompensated by even moderate MI. A vena contracta diameter ≥ 0.4 cm and a regurgitant area ≥ 0.2 cm2 are associated with increased mortality [16]. These observations justify the lower thresholds for defining severe MI when it is secondary.
Other important non-valvular elements are part of the quantitative assessment of secondary MI [38].
- Size of the LA; massive dilatation of the LA leads to dilatation of the mitral annulus; this is a poor prognostic factor.
- LV size and function: as the LV dilates, it becomes more spherical. This spherification is an important phenomenon in the distortion of the subvalvular apparatus. There are two different ways of calculating the sphericity index of the LV.
- Ratio of short-axis diameter (transgastric view) to long-axis length (2-cavity view 90° or long-axis 120°); normal value: < 0.7;
- Ratio of end-diastolic volume (Simpson's method) to the volume of a virtual sphere whose diameter is the distance between the plane of the mitral annulus and the apex of the LV; normal value: < 0.25.
- Anomaly of segmental kinetics; the extent of the ischaemic lesion is less important than its location in the vicinity of a papillary muscle.
Secondary MIs are particularly sensitive to the haemodynamic conditions of the patient, fluctuating with preload, afterload and ventricular function to a much greater extent than structural MIs. Under anaesthesia, it is therefore essential to assess them when the patient is balanced.
TEE examination before mitral valve surgery
The anaesthetist-echocardiographer must provide the surgeon with sufficient data to assess the chances of a successful operation. The pre-bypass TEE provides a dynamic view of the valve and allows the surgeon to assess whether the operation can be performed under satisfactory conditions. In particular, we are looking for some key data for the surgeon [27,28].
- Quantification of MI (tendency to underestimate in GA): PISA, vena contracta;
- Mechanism of MI: constriction, prolapse, cord rupture, dilatation;
- Exact location of origin of MI jet(s);
- Condition of the 2 commissures;
- Condition of annulus (calcifications) and subvalvular apparatus, dimensions of annulus;
- Mitral and pulmonary venous flow;
- LV function and size, ventricular remodelling, LA size;
- Specific measurements (see below): annular diameter, intercommissural distance, anterior leaflet length;
- In restrictive secondary MI; tentorial distance, systolic leaflet angle;
- Risk of dynamic obstruction of the LVOT after plasty (see Figure 11.49).
- Distance between the point of coaptation and the septum (C-sept) < 2.6 cm;
- Distance between coaptation point and annulus > 0.6 cm;
- Length of posterior leaflet > 1.5 cm;
- Length ratio anterior leaflet to posterior leaflet < 1.3;
- Mitroaortic angle < 140°.
The size of the prosthetic ring is chosen on the basis of the intertriginal distance, which is not modified by dilatation of the mitral annulus; in 2D TEE it is calculated by multiplying the length of the base of the anterior leaflet by 0.8 in the 60° bicommissural view with clockwise rotation of the probe; in 3D (en-face view) it is calculated in the same way from the intercommissural distance (see Figure 11.52A).
Certain echocardiographic criteria can be used to predict good feasibility and outcome of the procedure [26,28,45].
- Ring diameter < 5.0 cm (measured in mid-esophageal bicommissural 40-60° and long-axis 120-140° views);
- Anterior leaflet length > 3.0 cm, ratio of anterior leaflet length to annulus diameter > 0.65 (measured in diastole and long-axis view 120-140°);
- Lesion with < 3 festoons, stable commissures;
- Degree of MI moderate to severe but < massive;
- Single jet;
- Satisfactory subvalvular apparatus;
- No calcification of the annulus.
For restrictive secondary MI (IIIb), the criteria are slightly different (see Figure 11.80):
- Distance ring plane - coaptation point (tenting height) < 1.0 cm;
- Tenting area < 1.6 cm2;
- Anterior leaflet closure angle < 25°;
- LV sphericity index ≥ 1.5 (no significant dilatation).
Three-dimensional imaging provides a more accurate picture of the anatomy and motion of the mitral valve. In particular, it allows the size of the mitral annulus to be determined from measurements of the intercommissural distance, anterior leaflet height and annulus diameters (see Figures 11.74 and 11.78) [10,18,25,39].
Video: Three-dimensional view from the LA of prolapse of the anterior festoon of the posterior leaflet (P1) with rupture of the chord (on screen, lower left part of the valve).
Video: Three-dimensional view from the LA of a prolapse of the anterior festoon of the posterior leaflet (P2) with rupture of the chord (on screen, lower medial part of the valve).
Some data suggest that success is unlikely and recurrence is common (>50% of cases) [9,21,24,27,28].
- Involvement of ≥ 3 festoons;
- Involvement of 2 leaflets;
- Connected, poorly developed leaflets, commissural fusion (ARF, endocarditis);
- Length of anterior leaflet < 2.8 cm and posterior leaflet < 1.7 cm;
- Very large regurgitant orifice (> 0.4 cm2);
- Massive annular dilatation (diameter > 5.0 cm in structural MI and > 4.0 cm in functional MI) or very restrictive annulus (diameter < 3.5 cm);
- Extensive calcification.
In secondary ischaemic MI, annular diameter > 37 mm, tent height > 11 mm, tent area > 1.6 cm2 , anterior leaflet angle > 25°, posterior leaflet angle > 45° and severe colour jet MI are indicators of a 50% risk of recurrence [21]. Severe LV dilatation (end-diastolic diameter > 6.0 cm, telesystolic papillary muscle width > 2.5 cm) is also a handicap [28,33].
TEE examination after mitral valve surgery
The TEE image of the valve after plasty may appear bizarre because restoration of normal function is not synonymous with normal anatomy. In general, the posterior leaflet acts as a stop for the anterior leaflet, which opens and closes by itself (unicuspid valve). After bypass surgery, the echocardiographic criteria for successful mitral valve repair (see Figure 11.55) are defined as normal afterload (blood pressure), preload (blood volume) and ventricular function. The examination must be accompanied by an afterload test (MAP > 80 mmHg, PAsyst > 120 mmHg) and contractility stimulation to ensure that the results obtained correspond to real-life situations. The procedure is considered successful if the following conditions are met [13,29]
- Residual MI of degree ≤ mild; protosystolic and short residual MI.
- If the posterior leaflet is resected: the anterior leaflet lies under the posterior leaflet and the posterior part of the ring.
- If the 2 leaflets meet: coaptation author > 5-8 mm; this height is calculated by subtracting the distance between the mitral annulus and the coaptation point in systole from the total length of the anterior leaflet measured in diastole (120° long axis view) (see Figure 11.22).
- Ratio of coaptation height to anterior leaflet length > 0.2.
- Ratio of posterior leaflet length to anterior leaflet length < 0.4.
- Anterograde gradient: ΔPmax ≤ 4 mmHg, ΔPmoy ≤ 2 mmHg; the pressure half-time is not suitable for calculating the opening area, which is not circular.
- Vmax ≤ 1.5 m/s in the LVOT, no SAM (see Mitral plasty "HOCM effect" after MVR).
The echocardiographic indications to return to ECC (about 10% of cases) to complete the plasty or, failing that, to replace the valve are as follows [12,29].
- Structural defect that cannot be improved: perforation, dehiscence, leaflet tilt, ≥ minor para-annular leak;
- MI of degree ≥ mild to moderate:
- Non-adaptation of 2D or 3D images;
- Large, pansystolic MI jet;
- Presence of a ventricular PISA;
- Vena contracta > 0.3 cm;
- Regurgitant orifice > 0.25 cm2;
- Regurgitant volume > 25 mL;
- Restrictive stenosis: S < 2.0 cm2 , ΔPmax ≥ 10 mmHg, ΔPmoy ≥ 5 mmHg;
- SAM refractory to medical treatment (see Mitral plasty "HOCM effect" after MVR);
- The decision is based on the clinical context and not on the TEE image alone.
Measurement of gradient, effective area in diastole and regurgitant orifice area in systole is complex because the orifice area is not circular and the flow axis is oblique to the Doppler beam. Calculation by pressure half-time, reserved for valves with a surface area < 1.5 cm2 , is unsuitable for the irregular geometry of the orifice and the gap between the axis of diastolic flow and the Doppler axis. In these conditions, planimetry is more accurate after careful cropping of the 3D reconstruction. An excessive gradient may occur in the presence of fluid overload (too rapid transfer of ECC volume) or high cardiac output (excess catecholamines). These factors must be checked before deciding to return to ECC. Conversely, low cardiac output, severe diastolic dysfunction or associated aortic insufficiency may mask the reality by artificially reducing the diastolic gradient [12].
It is important to diagnose the mechanism of the residual insufficiency so that the surgeon can make an appropriate correction [13].
- Type I MI (central jet in the LA): ring too large, iatrogenic perforation, cleft;
- Type II MI (eccentric jet directed away from the prolapsing leaflet): residual prolapse, the location of which remains to be precisely defined;
- Type III MI (eccentric jet towards the prolapsing leaflet): excessive resection, malposition of the annulus, excessive shortening of the cords, ventricular dilatation;
- MI due to SAM: meso-tele-systolic jet, posterior leaflet too long, excess valve tissue, annulus too small.
When applying a valvuloplasty ring, it is important to check three things after weaning off bypass.
- Lateral wall contractility; the circumflex artery (CX) may be injured by the lateral fixation points.
- Sealing of the aortic valve; sutures at the base of the anterior leaflet can exert traction on the left cusp or non-coronal cusp of the aortic valve and cause an AI. They may also perforate an aortic leaflet.
- Integrity of the basal wall of the LV; decalcification of the annulus can lead to lesions in the atrioventricular groove; persistent presence of air in the LV and basal akinesia are warning signs of ventricular rupture.
| Mitral insufficiency on TEE |
| Definition of severe (chronic) MI (primary structural MI):
- Call sign: large regurgitant jet crossing the entire LA
- Dilatation of the LV (Dts > 4 cm/m2) and LA (> 5 cm)
- Non-coaptation of leaflets or tipping of a leaflet into the LA
- Regurgitant jet diameter at its origin > 0.7 cm
- Regurgitant orifice ≥ 0.4 cm2
- Regurgitant volume ≥ 60 mL
- Exercise-induced PAP > 60 mmHg
In acute MI, the left cavities are not dilated.
Severity criteria are more restrictive for severe secondary MI:
- Vena contracta diameter > 0.4 cm
- Regurgitant orifice > 0.2 cm2
- Regurgitated volume > 30 mL
- Ring diameter > 3.5 cm
- Tenting distance > 1.1 cm
- Tenting area > 2.5 cm2
Factors predicting good feasibility of mitral valve repair
- Ring diameter < 5.0 cm
- Anterior leaflet length to ring diameter ratio > 0.65
- Lesion with < 3 festoons, stable commissures
- Moderate to severe MI, single jet
- Satisfactory subvalvular apparatus
- Absence of annular calcification
Unfavourable criteria for surgical repair
- Involvement of ≥ 3 leaflets
- Involvement of 2 leaflets
- Anterior leaflet length < 2.8 cm and posterior leaflet length < 1.7 cm
- Regurgitant orifice > 0.4 cm2
- Massive annular dilatation or highly restrictive ring
- Extensive calcifications
- In secondary MI (ischaemia, LV dilatation): annulus height > 1.1 cm, annulus area > 2.5 cm2, ring diameter > 3.7 cm
Criteria for successful plasty
- Residual MI of degree ≤ mild
- Flattening of the anterior leaflet below the posterior leaflet
- Coaptation > 5-8 mm
- Ratio of coaptation height to anterior leaflet length > 0.2
- Ratio of posterior leaflet length to anterior leaflet length < 0.4
- Anterograde gradient: ΔPmax ≤ 4 mmHg, ΔPmean ≤ 2 mmHg
- Vmax ≤ 1.5 m/s in LVOT, no SAM
|
References
- ASHIKHMINA E, SHOOK D, COBEY F, et al. Three-dimensional versus two-dimensional echocardiographic assessment of functional mitral regurgitation proximal isovelocity area. Anesth Analg 2015; 120:534-42
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BORROW K, GREEN LH, MANN T, et al. End-systolic volume as a predictor of postoperative left ventricular performance in volume overload from valvular regurgitation. Am J Med 1980; 68:655-60.
- CARPENTIER A, CHAUVAUD S, FABIANI JN, et al. Reconstructive surgery of mitral valve incompetence. J Thorac Cardiovasc Surg 1980; 79:338-48
- CHERRY SV, JAIN P, RODRIGUEZ-BLANCO YF, FABBRO M. Noninvasive evaluation of native valvular regurgitation: a review of the 2017 American Society of Echocardiography Guidelines for the perioperative echocardiographer. J Cardiothorac Vasc Anesth 2018; 32:811-22
- COBEY FC, ASHIHKMINA E, EDRICH T, et al. The mechanism of mitral regurgitation influences the temporal dynamics of the vena contracta area as measured with color flow Doppler. Anesth Analg 2016; 122:321-9
- COBEY FC, FERREIRA R, URSPRUNG WW, et al. A novel approach to assess the three.dimensional anatomy of a mitral valve regurgitant orifice. J Cardiothorac Vasc Anesth 2017; 31:169-73
- COBEY FC, PATEL V, GOSLING A, URSPRUNG E. The emperor has no clothes: recognizing the limits of current echocardiographic technology in perioperative quantification of mitral regurgitation. J Cardiothorac Vasc Anesth 2017; 31:1692-4
- DEBONIS M, AL-ATTAR N, ANTUNES M, et al. Surgical and interventional management of mitral valve regurgitation: a position statement from the European Society of Cardiology working groups on Cardiovascular Surgery and Valvular Heart Disease. Eur Heart J 2016; 37:133-9
- ENDER J, EIBEL S, MUKHERJEE C, et al. Prediction of the annuloplasty ring size in patients undergoing mitral valve repair using real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr 2011; 12:445-53
- ENRIQUEZ-SARANO M, MILLER FA, HAYES SN, et al. Effective mitral regurgitant orifice area: clinical use and pitfalls of the proximal isovelocity surface area method. J Am Coll Cardiol 1995; 25:703-9
- ESSANDOHM. Intraoperative echocardiographic assessment of mitral valve area after degenerative mitral valve repair: a call for guidelines or recommendations. J Cardiothorac Vasc Anesth 2016; 30:1364-8
- FISCHER GW, ANYANWU AC, ADAMS DH. Intraoperative classification of mitral valve dysfunction: The role of the anesthesiologist in mitral valve reconstruction. J Cardiothorac Vasc Anesth 2009; 23:531-43
- FOSTER E. Mitral regurugitation due to degenerative mitral-valve disease. N Engl J Med 2010; 363:156-65
- GISBERT A, SOULIERE V, DENAULT AY, et al. Dynamic quantitative echocardiographic evaluation of mitral regurgitation in the operating department. J Am Soc Echocardiogr 2006; 19:140-6
- GRAYBURN PA, CARABELLO B, HUNG J, et al. Defining "severe" secondary mitral regurgitation. Emphasizing an integrated approach. J Am Coll Cardiol 2014; 64:2792-801
- GREWAL KS, MALKOWSKI MJ, PIRACHA AR, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesoophageal echocardiography. Am J Cardiol 2000; 85:199-203.
- GREWAL J, MANKAD S, FREEMAN WK. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009; 22:34-41
- HIEN MD, RAUCH H, LICHTENBERG A, et al. Real-time three-dimensional transesophageal echocardiography: improvements in intraoperative mitral valve imaging. Anesth Analg 2013; 116:287-95
- IZUMO M, SHIOTA M, KAR S, et al. Comparison of real-time three.dimensional transoesophageal echocardiography to two-dimensional transoesophageal echocardiographyfor quantification of mitral valve prolapse in patients with severe mitral regurgitation. Am J Cardiol 2013; 111:588-94
- KONGSAEREPONG V, SHIOTA M; GILLINOV AM, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol 2006; 98:504-8.
- LANCELLOTTI P, FATTOUCH K, LA CANNA G. Therapeutic decision-making for patients with fluctuating mitral regurgitation. Nat Rev Cardiol 2015; 12:212-9
- LANCELLOTTI P, MOURA L, AGRICOLA E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11:307-32
- LANCELLOTTI P, TRIBOUILLOY C, HAGENDORFF A, et al. Recommendations for the echocardiographic assessment of native valvular regurugitation: an executive summary from the EACI. Eur Heart J Cardiovasc Imaging 2013; 14:611-44
- LANG RM, BADANO LP, TSANG W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012; 13:1-46
- LEE APW, ACKER M, KUBO SH, et al. Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy. Circulation 2009; 119:2606-14
- MAHMOOD F, MATYAL R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg 2015; 121:34-58
- MASLOW A. Mitral valve repair: an echocardiographic review: Part I. J Cardiothorac Vasc Anesth 2015; 29:156-77
- MASLOW A. Mitral valve repair: an echocardiographic review: Part II. J Cardiothorac Vasc Anesth 2015; 29:439-71
- MATSUMARA Y, FUKUDA S, TRAN H. Geometry of the proximal isovelocity surface area in mitral regurgitation by 3-dimensional color Doppler echocardiography: difference between functional mitral regurgitation and prolapse regurgitation. Am Heart J 2008; 155:231-8
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017; 70:252-89
- NISHINO S, WATANABE N, KIMURA T, et al. Acute versus chronic ischemic mitral regurgitation. An echocardiographic study of anatomy and physiology. Circ Cardiovasc Imaging 2018; 11:e007028
- O'GARA PT, GRAYBURN PA, BADHWAR V, et al. 2017 ACC Expert consensus decision pathway on the management of mitral regurgitation. J Am Coll Cardiol 2017; 70:2421-49
- POELART JI, BOUCEZ S. Perioperative echocardiographic assessment of mitral valve regurgitation: a comprehensive review. Eur J Cardio-Thor Surg 2016; 50:801-12
- RYAN L, JACKSON B, PARISH L, et al. Quantification and localization of mitral valve tenting in ischemic mitral regurgitation using real-time three-dimensional echocardiography. Eur J Cardiothorac Surg 2007; 31:839-4
- SANFILIPPO F, JOHNSON C, BELLAVIA D, et sl. Mitral regurgitation grading in the operating room: a systematic review and meta-analysisi comparing preoperative and intraoperative assessments during cardiac surgery. J Cardiothorac Vasc Anesth 2017; 31:1681-91
- SHAKIL O, JAINANDUNSING JS, ILIC R, et al. Ischemic mitral regurgitation: an intraoperative echocardiographic perspective. J Cardiothorac Vasc Anesth 2013; 27:573-85
- SUGENG L, CHANDRA S, LANG RM. Three-dimensional echocardiography for assessment of mitral valve regurgitation. Curr Opin Cardiol 2009; 24:420-5
- SUGIMOTO T, DULGHERU R, MARCHETTA S, et al. What does 3D echocardiography ass to 2D echocardiography in the assessment of mitral regurgitation? Curr Cardiol Rep 2017; 19:90
- TAN TC, ZENG X, JIAO Y, et al. Three-dimensional field optimization method: clinical validation of a novel color Doppler method for quantifying mitral regurgitation. J Am Soc Echocardiogr 2016; 29:926-34
- TRICHON BH, FELKER GM, SHAW LK, et al. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 2003; 91:538-43
- VUILLE C, WEYMAN AE. Left ventricle I: general considerations, assessment of chamber size and function. In: WEYMAN AE (ed). Principles and practice of echocardiography. Philadelphia: Lea & Febiger, 1994, 575-624.
- WIERUP P, NIELSEN SL, EGELBLAD H, et al. The prevalence of moderate mitral regurgitation in patients undergoing CABG. Scand Cardiovasc J 2009; 43:46-9
- YAMAUCHI T, TANIGUCHI K, KUKI S, et al. Evaluation of the mitral valve leaflet morphology after mitral valve reconstruction with a concept of "coaptation length index". J Card Surg 2005; 20:432-5
- ZOGHBI WA, ADAMS D, BONOW RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the ASE developped in collaboration with the SCMR. J Am Soc Echocardiogr 2017; 30:303-71