The new anti-Xa and anti-thrombin oral anticoagulants (NOACs) have a lower bleeding risk than AVKs: reduction in the average incidence of major bleeding from 2% (AVKs) to 1% (NOACs), reduction in intracranial bleeding by 51% and mortality by 40% [36]. However, risk of bleeding remains 1-3.6% per year depending on substance and indication [5]. If severe bleeding on anticoagulants threatens the survival of the patient or if the urgency of the surgery prevents the time required for spontaneous elimination of the substance from being respected, it is possible to reverse the effect of anticoagulants, either with a specific antidote or with coagulation factors. This is not without risk, and the way this is done must take into account several important considerations [41].
- The risk of bleeding injury. Major bleeding on anticoagulation is considered to be a drop in Hb of 20-50 g/L, blood loss requiring transfusion of >2 units of blood or intracranial haemorrhage [34].
- The bleeding risk of surgery. Most operations can be performed safely despite a residual anticoagulant effect. Only procedures with a high bleeding risk require surgery under normalized coagulation conditions. These are mainly: intracranial or intra spinal neurosurgery, haemorrhagic procedures with difficult haemostasis, major orthopaedic or oncological surgery, major urogenital surgery (because of endogenous urokinase) [9].
- The thrombo-embolic risk of the disease. If it is high, complete reversal of anticoagulation is always dangerous. In this situation, the aim is not to restore normal coagulation, but to reduce anticoagulation to a level compatible with the bleeding risk of the injury or surgery. Prophylactic reversal of anticoagulation is not recommended except in operations with a very high bleeding risk in patients with a low thromboembolic risk [44].
- The thrombotic risk of surgery. Surgery comes along with hypercoagulability, platelet activation and inhibition of fibrinolysis which increase the risk of vascular thrombosis. A history of recent vascular events and cancerous diseases are at particularly high risk. Thrombosis is 6 times more common in cancer surgery than in general or vascular surgery [10,11,43].
- Thrombotic risk of the agents used for antagonism. Activated prothrombin complexes and factor rVIIa can cause arterial thrombosis in 1-20% of cases [21,26].
- The degree of emergency. If response time is too short for the effect to wear off on its own, anticoagulation can be reversed by an antidote, if available. Otherwise, non-specific clotting factors can be used.
- Difficult situations should be dealt with a case-by-case basis and the help of an haematologist.
Conventional laboratory tests are poor at judging the degree of anticoagulation on NOACs (see Table 8.4) [22,40,45]. TT and TP are sensitive to dabigatran and xabans respectively, but the correlation is by no means quantitative and normal values do not exclude significant residual levels [37]. PTT is less sensitive than TT to dabigatran, and PT is less sensitive to apixaban than to other xabans [7]. The specific tests for dabigatran are the diluted thrombin time (dTT) and the ecarin clotting time (ECT); for xabans, these are the calibrated anti-Xa tests for each agent [6]. A residual level ≤ 30 ng/mL of dabigatran, rivaroxaban or apixaban, measured >4 hours after the last drug dose, is considered to be safe for surgery [12,31]. Unfortunately, specific tests are not always available in emergency. As the half-life of NOACs is short (on average 8-12 hours), simply waiting is the first measure to be considered if situation permits [5,18,19]. The longer one waits, the lower the risk of bleeding after 24 hours, plasma level is only 25%, and a delay of 48 hours guarantees no effect (residual level of 6-7%). As the elimination of NOACs is largely renal, this delay should be doubled when creatinine clearance is < 50 mL/min. In non-elective life-saving procedures, surgery can be considered as soon as the anti-Xa assay corresponds to a plasma level of < 50 ng/mL [12]. Management always come along with general measures: local compression, surgical or endoscopic haemostasis, radio-interventional embolisation, volume administration, possibly blood transfusions in case of acute anaemia or platelets in case of thrombocytopenia. Anticoagulant antagonism can be achieved either by specific antidotes when they exist, or by non-specific drugs and coagulation factors.
Specific antidotes
Conventional anticoagulants have an antidote that reverses their effect completely.
- Non-fractionated heparin (UFH): protamine. One mg of protamine (100 IU) neutralises 1 mg of heparin (100 IU). Usually, a dose of protamine is given that is 80% of heparin dose.
- Low molecular weight heparins (LMWH): protamine is a partial antagonist. A neutralisation of 60% of the anticoagulant effect is possible with 1 mg protamine per 100 IU of anti-Xa effect (maximum dose: 50 mg), provided it is within 8 hours of administration [6,14].
- Anti-vitamin K (AVK) agents: intravenous vitamin K (Konakion® ) (2.5-5.0 mg, possibly 10 mg) administered over > 20 minutes; maximum effect after12-24 hours [3,10].
Development of specific antidotes for NOACs is progressing rapidly. Currently three exist, one of which is available [4,30,37].
- Idarucizumab (Praxbind® , currently marketed); monoclonal antibody that binds irreversibly with dabigatran (350 times greater affinity than thrombin). Despite its short half-life, it neutralises all circulating dabigatran. Normalization of TT and ECT, surgical haemostasis normalized in 94% of cases, time to emergency surgery 1.6 hours [33,38].
- Start of action: < 5 minutes.
- Half-life: initial 47 min, terminal 10.3 hours; renal elimination.
- Dosage: 2 x 2.5 g iv in 10 min at 15 min intervals.
- As circulating dabigatran is neutralised, dabigatran distributed in the extracellular fluid and tissues re-diffuses into the plasma and requires a new dose of antagonist; this may occur several times.
- Andexanet alfa (Andexxa® ); functions as a factor Xa decoy and sequesters factor Xa inhibitors (xabans) in a 1:1 stochiometric ratio. Anti-Xa activity is reduced by 92-94% in 5 minutes and the effect lasts for 2 hours [39]; in a series of 352 patients with an average anti-Xa factor level of 150 ng/mL and major intracranial or gastrointestinal bleeding, haemostasis is effective in 82% of cases; anti-Xa factor is lowered to an average of 11 ng/mL [8]. Duration of action of andexanet is shorter than that of xabans, so there is a risk of rebound effect of anticoagulation; administration must then be repeated [28]. The substance is being marketed to reverse the effect of rivaroxaban, apixaban and edoxaban; approved by FDA in April 2018, and now by the EMA (November 2022).
- Start of action: 2 minutes.
- Half-life: initial 1 hour, terminal 6 hours.
- Dose 1: 400 mg (bolus iv at 30 mg/min), then 2 hour infusion at 4 mg/min (480 mg) for apixaban, or for rivaroxaban at > 7 hours from last dose.
- Dose 2: 800 mg followed by a 2-hour infusion at 8 mg/min (960 mg) for edoxaban, or for rivaroxaban at < 7 hours from the last dose.
- Ciraparantag (aripazine, PER977); a versatile molecule containing 8 covalent H+-binding sites , effective in reversing the effect of xabans, argatroban, fondaparinux, dabigatran and heparins. Clotting time is normalised within 10 minutes [3], and bleeding is reduced by 90% in animals [25]. As clinical trials are not yet complete, the substance will not be on the market for 1-2 years.
- Start time: 5-10 minutes.
- Duration of action: 24 hours.
- Dosage: 100-300 mg bolus iv.
Indications for these antidotes are life-threatening bleeding, particularly intracranial haemorrhage, and urgent major surgery that cannot be postponed by 12-24 hours. There is almost no danger of secondary thrombosis with them because they have no effect on coagulation factors [5]. As long as they are available, they are a better choice to non-specific agents, but their indications will be limited to uncontrollable situations. They have no indication in the case of overdose of a NOAC if the patient is not bleeding, nor beyond 24 hours after the last dose because the circulating level is too low [47]. They completely inhibit the circulating substance, but bleeding may resume after a few hours due to blood re-diffusion of the anticoagulant from the extravascular compartment. Although laboratory tests become normal and effectively reduce bleeding, these antidotes have a relative effect on patients survival, who suffer a high mortality related to the basic pathology justifying anticoagulation and to the consequences of the haematoma, particularly intracranial. Moreover, the outcome of patients hospitalised in emergency for haemorrhage on NOACs, without antidote , is similar to that of patients bleeding on AVKs, for which the antidote is in common use [40]. This, together with their extremely high cost, limits the indications for antagonists to a niche area.
Non-specific antagonism
Without antidote, non-specific reversal must make sure that patient's blood calcium, acid-base balance and temperature are normal (see Clotting factors) [21,29,37]. The effects of anticoagulation can be mitigated by adding two substances [15,35,41,46].
- Tranexamic acid (2 gm iv); this antifibrinolytic may inhibit plasmin production and excessive activation of fibrinolysis. It is relatively effective in situations such as major traumatic haemorrhage, ECC or postoperative inflammatory syndrome (see Antifibrinolytics).
- Desmopressin (0.3 mcg/kg iv) stimulates production of factor VIII and von Willebrand factor by the endothelium and improves platelet adhesion (see Platelets).
Without improvement, next step is to administer clotting factors. Unfortunately, in case of NOACs, the circulating anticoagulant also blocks the administered factors, making them much less effective than AVKs [13,18,19,30,41,47].
- Prothrombin complex concentrate (PCC); contains factors II, IX, X (3-factor preparations) and factor VII (4-factor preparations), as well as protein C and protein S. Dosage: 25-50 IU/kg. Partially reverses the effect of xabans on coagulation tests in vitro and in vivo, but ineffective on dabigatran [16,27,49]. Four-factor preparations are preferred; the effective dose is 50 IU/kg [49]. The half-lives of the different factors are not identical and range from 5 hours for FVII to 60 hours for FII.
- Activated prothrombin complex (aPCC) or FEIBA® (Factor eight inhibitor bypass activity), contains factors II, IX, X and VII, a large proportion of which are in activated form. Dosage: 30-50 IU/kg. A good alternative to dabigatran, it is also more effective than PCCs in reversing rivaroxaban in vitro, but it carries a significant risk of secondary thrombosis [32].
- Activated factor VII (rFVIIa, Novo-Seven® ), 90 mcg/kg; rFVIIa activates factor X to Xa, which generates more thrombin. It may be indicated to counteract anti-Xa. Although it normalises coagulation tests, rFVIIa does not significantly reduce blood loss [16]. It has a high risk of thrombosis [1]. Although it may be useful in cases of uncontrollable bleeding on fondaparinux [23], rVIIa has no success in reversing the effect of NOACs, and should no longer be used in this situation.
- The order of increasing effectiveness in reversing effect of xabans on coagulation tests is: rFVIIa < PCC < aPCC; however, the effect is limited and does not exceed 50% correction [32].
- Fresh frozen plasma: there is no evidence that FFP reverses the effect of NOACs; its main use is to restore circulating volume in addition to transfusions [13,18,47].
- Platelet transfusion in case of thrombocytopenia or associated antiplatelet therapy.
A French registry of 732 patients with severe bleeding on NOACs showed that FFP was used in 38% of cases with an apparent success rate on haemostasis of about 40%, but with no significant reduction in mortality [2]. FEIBA and rFVIIa are reserved for potentially lethal blood loss that is no longer responsive to haemostasis and supportive measures, but offer no guarantee of success [47]. The evidence for efficiency of clotting factors in NACO bleeding is very limited and inconclusive, particularly for rFVIIa [5]. These agents should not be used prophylactically, nor in cases of supratherapeutic anticoagulant levels without uncontrolled active bleeding, as danger of secondary thrombosis is real [37]. Fresh frozen plasma (FFP) is unlikely to be effective because it contains low concentrations of factors and there is a risk of haemodynamic overload to achieve the desired effect (1-2 litres of FFP to normalise the factor levels). The aim here is not to compensate for factor depletion, as in the reversal of AVKs, but to overcome their inhibition by a circulating substance [1,20,21]. In this context, FFP is only useful as a plasma expander. Reversal of anticoagulation with NOACs is prohibitively expensive: a dose of activated PCC (50 IU/kg) is more than CHF 5,000, and Praxbind® is CHF 3,400 [37].
These treatments are based on persistent bleeding and alterations in coagulation tests (PT, aPTT, TT, thromboelastography, anti-Xa). In the absence of heparin, an anti-Xa value < 0.1 U/mL suggests that the effect has disappeared. A residual level < 50 ng/mL of xaban or dabigatran is compatible with general surgery, and a level ≤ 30 ng/mL allows surgery in situations with high bleeding risk [12,41]. Thromboelastogram is probably the most informative test. Although most standard tests are improved, their eventual normalisation by treatment unfortunately does not ensure that haemostasis is normal during surgery [42]. On the other hand, providing coagulation factors may improve bleeding balance without significantly altering laboratory tests [46]. Coupling between laboratory values and clinical bleeding is therefore quite loose [13].
If the anticoagulant effect cannot be reversed, an attempt can be made to slow down the digestive absorption of the product with activated charcoal (30-50 g) if the last intake was less than 3-6 hours ago. The substance can also be removed by filtration [17,47]:
- Hemodialysis for dabigatran, as its protein binding is modest (35%); 40-65% of the substance is eliminated in 4 hours, but a rebound effect may occur when dialysis is stopped [24]. The initiation of haemodialysis can be chaotic if haemorrhagic shock sets in.
- By plasmapheresis for rivaroxaban and apixaban, which are too strongly bound to plasma proteins (80-95%) to be effectively dialysed.
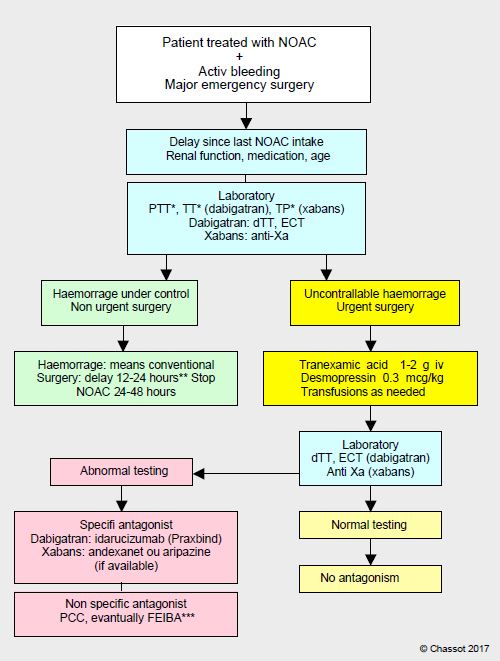
However, this option is more theoretical than realistic. Setting up haemodialysis or plasmapheresis with a heavily bleeding patient is uncertain, and central venipuncture under anticoagulation carries obvious risks of potentially disastrous bleeding. In case of a bleeding patient on anticoagulants, elevated PT and TT, and possibly PTT, should raise suspicion of NOAC use and trigger an anti-Xa test and dTT (Hemoclot™) as check. Repeated at 6 hour intervals if clinical condition permits, two successive assays allow the patient to be plotted on the elimination curve of the substance and decide if antagonism is required. Surgery can be considered as soon as the anti-Xa or anti-IIa assay is < 50 ng/mL, which can be achieved by simply waiting if situation permits. It is essential to know the time elapsed since last dose of the drug, which allows one to know whether the circulating concentration is at its peak or nadir, the age and renal function of the patient, which allows one to assess the rate of elimination of the product, as well as his or her comorbidities and co-medications, which allow one to judge possible interference. An algorithm is used to clarify the sequence of the various therapeutic measures (Figure 8.14).
Figure 8.14: Algorithm for the management of patients with bleeding or requiring urgent bleeding surgery when using the new oral anticoagulants (NOACs): dabigatran, rivaroxaban, apixaban or edoxaban.
*: These tests have a warning value, but have no quantitative value; in particular, a normal value does not exclude the presence of a significant level of the substance. **The half-life of NOACs is on average 12 hours; after24 hours, the plasma level is 25%, and a delay of 48 hours guarantees no effect (residual level of 6-7%). Standard surgery is possible as soon as the anti-Xa assay corresponds to a plasma level < 50 ng/mL, which can be achieved by a simple wait if the situation allows. Bleeding risk surgery requires a level of < 30 ng/mL. ***: activated PCC (FEIBA) and rFVVa are reserved for life-threatening bleeding.
Conventional means: local compression and surgical or endoscopic haemostasis, radio-interventional embolisation, volume administration (crystalloids, plasma expanders), activated charcoal if ingested < 4 hours. dTT: diluted thrombin time (Hemoclot™). ECT: ecarin coagulation time.
| Reversal of the effect of anticoagulants |
|
The aim is to achieve a level of anticoagulation that is an acceptable compromise between spontaneous or intraoperative blood loss and the danger of thromboembolism. Antagonising anticoagulation does not make sense outside of major bleeding situations. Prophylactic reversal of anticoagulation is not recommended except in operations with very high bleeding risk in patients with low thromboembolic risk.
Specific antidotes:
- Non-fractionated heparin: protamine (1 mg protamine to 1 mg heparin)
- LMWH: protamine is a partial antagonist
- Anti-vitamin K agents: vitamin K (2.5-5 mg iv / 12 h), prothrombin complex if
emergency
- Dabigatran: idarucizumab (Praxbind® )
- Other NOACs: antidote (andexanet alfa and aripazine) in the pipeline
Non-specific agents :
- Tranexamic acid (2 gm, 15 mg/kg)
- Desmopressin (0.3 mcg/kg)*
- Prothrombin complex (factors II, IX, X and a variable dose of factor VII)**
- Activated prothrombin complex (aPCC), FEIBA® (factors II, IX, X and VII partially
activated)***
- Activated Factor VII (rFVIIa, Novo-Seven® ): lifesaving in the event of imminent death ****
- Order of effectiveness in reversing the effect of rivaroxaban on in vitro coagulation tests:
PCC < rFVIIa < aPCC
- Transfusion of blood for acute anaemia and platelets for thrombocytopenia
* : preferably in cases of platelet dysfunction and FVIII or vWF deficiency
**: preferably to counteract anti-Xa agents (rivaroxaban, apixaban, edoxaban)
*** : preferably to counteract anti-thrombin agents (dabigatran)
****: the effectiveness of rFVIIa is not proven in this setting.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- AGENO WA, GALLUS AS, WITTKOWSKY A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e44S-e88S
- ALBALADEJO P, SAMAMA CM, SIÉ P, et al. Management of severe bleeding in patients treated with direct oral anticoagulants. An observational registry analysis. Anesthesiology 2017; 127:111-20
- ANSEL JE, BAKHRU SH, LAULICHT BE, et al. Use of PER977 to reverse the antocoagulant effect of edoxaban. N Engl J Med 2014; 371:2141-2
- ARBIT B, NISHIMURA M, HSU JC. Reversal agents for direct oral anticoagulants: a focused review. Int J Cardiol 2016; 223:244-50
- ARONIS K, HYLEK EM. Who, when, and how to reverse non-vitamin K oral anticoagulants. J Thromb Thrombolysis 2016; 41:253-72
- BROWN C, JOSHI B, FARADAY N, et al. Emergency cardiac surgery in patients with acute coronary syndromes: a review of the evidence and perioperative implications of medical and mechanical therapeutics. Anesth Analg 2011; 112: 277-99
- BROWN KS, ZAHIR H, GROSSO MA; et al. Nonvitamin K antagonist oral anticoagulant activity: challenges in measurement and reversal. Critical Care 2016; 20:273
- CONOLLY SJ, CROWTHER M, EIKELBOOM JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med 2019; 380:1326-35
- DOUKETIS JD. Perioperative management of patients who are receiving warfarin therapy: an evidence-based and practical approach. Blood 2011; 117:5044-9
- DOUKETIS JD, BERGER PB, DUNN AS, et al. The perioperative management of antithrombotic therapy. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008; 133:299S-339S
- DOUKETIS JD, SPYROPOULOS AC, SPENCER FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, Chest 2012; 141 (2 suppl): e326S-50S
- DROUET L, BAL DIT SOLLIER C, STEINER T, et al. Measuring non-vitamin K antagonist oral anticoagulant levels: When is it appropriate and which methods should be used? Int J Stroke 2016; 11:748-58
- EIKELBOOM JW, KOZEK-LANGENECKER S, EXADACTYLOS A, et al. Emergency care of patients receiving non-vitamin K antagonist oral anticoagulants. Br J Anaesth 2018; 120:645-56
- GARCIA DA, BAGLIN TP, WEITZ JI, et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e24S-e43S
- GHADIMI K, LEVY JH, WELSBY IJ. Perioperative management of the bleeding patient. Br J Anaesth 2016; 117(suppl 3):iii18-iii30
- GODIER A, MICHOT A, LE BONNIEC B, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology 2012; 116:94-102
- HANKEY GJ, EIKELBOOM JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation 2011; 123: 1436-50
- HEIDBUCHEL H, VERHAMME P, ALINGS M, et al. European Heart Rythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015; 17:1467-507
- HEIDBUCHEL H, VERHAMME P, ALINGS M, et al. Updated European Heart Rythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Executive summary. Eur Heart J 2017; 38:2137-49
- HOLBROOK A, SCHULMAN S, WITT DM, et al. Evidence-based management of anticoagulant therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e152S-e184S
- KAATZ S, KOUIDES PK, GARCIA DA; et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87:S141-S145
- KOVAKS RJ, FLAKER GC, SAXONHOUSE SJ, et al. Practical management of anticoagulation in patients with atrial fibrillation. J Am Coll Cardiol 2015; 65:1340-60
- KOZEK-LANGENECKER SA, AHMED AB, AFSHARI A, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. First update 2016. Eur J Anaesthesiol 2017; 34:332-95
- KUMAR R, SMITH RE, HENRY BL. A review and recommendations for the management of patients with life-threatening dabigatran-associated hemorrhage: A single-center university hospital experience. J Intensive Care Med 2015; 30:462-72
- LAULICHT B, BAKHRU S, JIANG X. Antidote for new oral anticoagulants: mechanism of action and binding specificity of PER977. J Thromb Haemost 2013; 11:75
- LEVI M, LEVY JH, ANDERSEN HF, et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med 2010; 363: 1791-800
- LEVI M, MOORE KT, CASTILLEJOS CF, et al. Comparison of three-factor and four-factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost 2014; 12:1428-36
- 28LU G, DEGUZMMAN FR, HOLLENBACH SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013; 19:446-53
- MAJEED A, SCHULMAN S. Bleeding and antidotes in new oral anticoagulants. Best Pract Res Clin Haematology 2013; 26:191-202
- MAR PL, FAMILTSEV D, EZEBOWITZ MD, et al. Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: Review of the literature and recommendations for specific populations and procedures. Int J Cardiol 2016; 202:578-85
- MUECK W, SCHWERS S, STAMPFUSS J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subject, specific patient populations and relevance of coagulation monitoring. Thrombosis Journal 2013; 11: 10
- PERZBORN E, HEITMEIER S, LAUX V, BUCHMULLER A. Reversal of rivaroxaban-induced anticoagulation with prothrombin complex concentrate, activated prothrombin complex concentrate and recombinant activated factor VII in vitro. Thrombos Res 2014; 133:671-81
- POLLACK CV, REILLY PA, VAN RYN J, et al. Idarucizumab for dabigatran reversal - Full cohort analysis. N Engl J Med 2017; 377:431-41
- PURRUCKER JC, STEINER T. Management of acute stroke in patients on oral anticoagulants. Curr Op Neurol 2017; 30:1-7
- RAMIREZ RJ, SPINELLA PC, BOCHICCHIO GV. Tranexamin acid update in trauma. Crit Care Clin 2017; 33:85-99
- RUFF CT, GIUGLIANO RP, BRAUNWALD E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet 2014; 383:955-62
- RUFF CT, GIUGLIANO RP, ANTMAN EM. Management of bleeding with non-vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation 2016; 134:248-61
- SCHIELE F, VAN RYN J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013; 121:3554-62
- SIEGAL DM, CURNUTTE JT, CONNOLLY SJ, et al. Andexanet alfa for reversal of factor Xa inhibitor activity. N Engl J Med 2015; 373:2413-24
- SINGER AJ, QUINN A, DASGUPTA N, et al. Management and outcomes of bleeding events in patients in the emergency department taking warfarin or a non-vitamin K antagonist oral anticoagulant. J Emerg Med 2017; 52:1-7
- SPAHN DR, BEER JH, BORGEAT A, CHASSOT PG, et al. New oral anticoagulants in anesthesiology. Transf Med Hemother 2019; 46:282-93
- SPAHN DR, KORTE W. Novel oral anticoagulants. New challenges for anesthesiologists in bleeding patients. Anesthesiology 2012; 116:9-11
- TAFUR AJ, WYSOKINSKI WE, McBANE R, et al. Cancer effect on periprocedural thromboembolism and bleeding in anticoagulated patients. Ann Onc 2012; 23:1998-2005
- TOTH P, MAKRIS M. Prothrombin complex concentrate-related thrombotic risk following anticoagulation reversal. Thromb Haemost 2012; 107:599
- TUMMALA R, KAVTARADZE A, GUPTA A, GHOSH RK. Specific antidotes against direct oral anticoagulants: A comprehensive review of clinical trials data. Int J Cardiol 2016; 214:292-8
- WEBER CF, DIETRICH W, SPANNAGL M, et al. A point-of-care assessment of the effects of desmopressin on impaired platelet function using Multiple Electrode whole-blood Aggregometry in patientsaftercardiac surgery. Anesth Analg 2010; 110:702-7
- WEITZ JI, POLLACK CV. Practical management of bleeding in patients receiving non-vitamin K antagonists oral anticoagulants. Thromb Haemost 2015; 114:1113-26
- WEITZ JI, QUINLAN DJ, EIKELBOOM JW. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation 2012; 126:2428-32
- ZAHIR H, BROWN KS, VANDELL AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by 4-factor prothrombin complex concentrate. Circulation 2015; 131:82-90