The usual coagulation tests are part of the evaluation of the anticoagulated patient preoperatively, but have limited significance in predicting bleeding risk, because they essentially assess in vitro coagulation, not the effect on circulating cells (platelets, leukocytes) or the endothelium. Standardisation is difficult [9]. Their sensitivity depends on the reagent used [2,16].
- The aPTT (activated partial thromboplastin time) is sensitive to factors I, II, V, X (common pathway) and VIII, IX, XI, XII (intrinsic pathway); the pathological threshold is empirically set at ≥ 60 seconds (1.5-1.8 times the normal value); the normal value (30-40 sec) is variable according to the laboratory.
- PT (prothrombin time) is sensitive to factors I, II, V, VII, X (extrinsic pathway); it measures vitamin K dependent factors. The pathological threshold is empirically set at ≥ 40 seconds. The International normalized ratio (INR) is a way of expressing PT that takes into account the sensitivity of the reagent used for the test (thromboplastin) and reduces inter-laboratory variation; it is specific to anti-vitamin K agents and does not apply to other substances. The normal INR is < 1.5.
- TT (thrombin time) measures the conversion of fibrinogen to fibrin and is prolonged by thrombin inhibitors such as heparin, bivalirudin or dabigatran.
- Fibrinogen must remain > 2 gm/L to allow adequate fibrin polymerisation and avoid excessive bleeding. The Clauss method usually used for its measurement is sensitive to the presence of colloids, which leads to an overestimation of its level in their presence. Neither the Clauss technique nor the FIBTEM of thromboelastography are standardised.
- Thrombocyte levels are not predictive of function, but must be > 70,000/mcL to ensure an effective thrombus.
Monitoring of anticoagulants
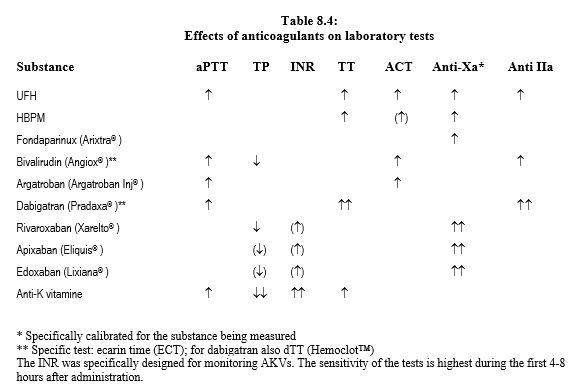
Laboratory tests are essential for monitoring patients on heparin or anti-vitamin K (AVK), because the response to these drugs is highly variable between individuals and according to the circumstances (diet, co-medication, etc). This is not the case for the new oral anticoagulants (NOACs), which were developed precisely with the aim of offering a linear response between dose and effect, and avoiding need for frequent laboratory assays. As a result, reliable monitoring is not available for these substances, which tend to alter tests in a non-specific manner. For NOACs, it is difficult to assess with certainty the actual degree of anticoagulation in a patient and the haemostatic threshold at which coagulation can be considered satisfactory. Laboratory tests are affected differently by these different anticoagulants (Tables 8.4 and 8.11), and the safety thresholds for INR or aPTT validated for AVKs do not apply to the new substances [3,14].
It is usual to assess the effect of non-fractionated heparin (UFH) by two laboratory tests, as the substance has non-linear pharmacokinetics [7].
- The activated partial thromboplastin time (aPTT) performs well in the usual assays; the target value is 2 to 3 times the control value.
- ACT (activated clotting time) is preferred for high dosages as in ECC; value sought: ≥ 450 seconds for a standard ECC circuit, 250-300 seconds for a heparin coated circuit.
The activity of LMWHs, which have little effect on aPTT, fondaparinux and anti-Xa substances (fondaparinux, xabans) is measured by measuring the calibrated anti-Xa effect for the substance, which peaks 3-5 hours after administration. The target value is 0.5-1.0 or 1.0-2.0 IU/mL for prophylactic or therapeutic administration, respectively [11]. This assay is not required for patient monitoring, except in cases of obesity, pregnancy, renal failure, overdose, bleeding or urgent preoperative decisions [4].
The variability of individual response and the effects of diet or co-medication mean that the effect of anti-viatmin K should be monitored by thromboplastin time (TP or PT) or INR. The desired therapeutic level is ≤ 30% for PT and 2.5 - 3.0 for INR [1]. Coagulation tests are variably affected by NOACs, especially as the different reagents used for each test differ considerably in their sensitivity to these anticoagulants (see Table 8.4) [4,5,6,8,10,13,17].
- The TT (thrombin time) is hypersensitive to dabigatran; a normal TT excludes the presence of dabigatran. This excess sensitivity is corrected by the diluted TT (dTT) and calibrated for dabigatran (Hemoclot™), which allows a quantitative determination with an excellent correlation to plasma levels (r2 = 0.92-0.99) [4]. The TT is insensitive to xabans.
- The aPTT is sensitive to dabigatran but non-linearly; it allows determination of the likely presence or absence of anticoagulant effect, but not quantification. A residual effect of the substance is possible even if the aPTT is normal. The aPTT is not correlated with xabans.
- PT is sensitive to direct anti-Xa agents (rivaroxaban, apixaban, edoxaban), but insensitive to direct anti-IIa agents (dabigatran). It is partially correlated with the plasma level of rivaroxaban, but very dependent on the reagent used. It correlates less well with the level of edoxaban and very little with the level of apixaban. It is only reliable for rivaroxaban if performed with a xaban-sensitive thromboplastin (Neoplastin Plus™, sensitive to levels ≥ 50 ng/mL). In the absence of standardisation between different reagents, PT cannot be considered to predict the intensity of anticoagulation. A normal PT does not exclude residual anti-Xa activity [10,13].
- Chromatographic assays measuring calibrated anti-Xa activity for each substance allow the effect of direct (rivoraxaban, apixaban, edoxaban) and indirect (LMWH, fondaparinux) anti-Xa drugs to be quantified in IU/mL; their results correlate closely with the serum levels of these substances measured by mass spectrography (r2 = 0.95-1.0) [4] ], although the correlation breaks down somewhat < 50 ng/mL and > 200 ng/mL [15]. These are the preferred tests for xabans. However, there are no pharmacological data on the relationship between laboratory results and patient bleeding risk [13].
- The ecarin clotting time (ECT) measures thrombin time with a derivative of viper venom as a prothrombin activator and is suitable for the quantitative evaluation of dabigatran and bivaluridine (r2 = 0.92-1.0) [4].
A normal PT or aPTT indicates that residual activity of rivaroxaban or dabigatran, respectively, is likely to be low, but does not by itself allow surgery [12]. In a patient who is bleeding spontaneously or excessively in relation to the lesion, a PT of 40-50% and/or a prolonged TT should raise the suspicion of NOAC therapy; a dTT and anti-Xa test will confirm or refute this. The values obtained by the different tests depend on time between the examination and the last intake of the drug; they are highest during the first few hours (peak serum concentration), when the tests have their maximum sensitivity [13]. It is therefore recommended that an elective measurement is performed in the residual period, i.e. between 12 and 18 hours after the last intake, to get an idea of the minimum concentration of the substance to which the patient is still exposed [10,13]. Identification and exact measurement of the concentration of these substances is possible by high performance liquid chromatography or mass spectrography, but these tests are not universally available and are mostly not refunded. Because of the dependence of NOACs on renal excretion, regular monitoring of creatinine clearance is recommended in patients taking dabigatran or xabans.
| Monitoring of anticoagulants |
|
Monitoring of patients on infusion of non-fractionated heparin (UFH) :
- Thromboplastin time (aPTT, activated partial thromboplastin time); value 1.5 - 2.5 times the control value
- ACT (activated clotting time); preferable at high doses; ≥ 450 seconds in ECC
Monitoring of patients on LMWH, fondaparinux and anti-Xa: calibrated anti-Xa assay for the substance. Target value: 0.5-1.0 IU/mL (prophylactic) or 1.0-2.0 IU/mL (therapeutic).
Monitoring of patients on anti-vitamin K agents (AVK) :
- PT (prothrombin time), target value: ≤ 30%.
- INR (International normalized ratio), target value: 2.5 - 3.0
Monitoring of patients on new oral anticoagulants (NOACs) :
- TT, aPTT and ECT extended with dabigatran; Hemoclot™ (quantitative)
- Prolonged PT with rivaroxaban and edoxaban (not reliable for apixaban)
- Calibrated anti-Xa activity for the substance as a quantitative measure for xabans
- TP and aPTT are non-quantitatively prolonged; their normality does not allow to rule out anticoagulant activity of xabans or dabigatran, respectively
- The serum levels obtained with the same assay are highly variable between patients and do not necessarily reflect the same level of risk do not appear to be correlated with bleeding risk, which is multifactorial.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- AGENO WA, GALLUS AS, WITTKOWSKY A, et al. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e44S-e88S
- BONHOMME F, FONTANA P. Laboratory testing of hemostasis. In: MARCUCCI C, SCHOETTKER P, editors. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer Verlag, 2015
- CHASSOT PG, BARELLI S, BLUM S, et al. Antiplatelet therapy and anticoagulation. In: MARCUCCI C, SCHOETTKER P, editors. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer Verlag, 2015
- CUKER A, SIEGAL DM, CROWTHER MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014; 64:1128-39
- DROUET L, BAL DIT SOLLIER C, STEINER T, et al. Measuring non-vitamin K antagonist oral anticoagulant levels: When is it appropriate and which methods should be used? Int J Stroke 2016; 11:748-58
- GALANIS T, THOMSON L, PALLADINO M, et al. New oral anticoagulants. J Thromb Thrombolysis 2011; 31:310-20
- GARCIA DA, BAGLIN TP, WEITZ JI, et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e24S-e43S
- HANKEY GJ, EIKELBOOM JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation 2011; 123: 1436-50
- KOZEK-LANGENECKER SA. Perioperative coagulation monitoring. Best Pract Res Clin Anaesthesiol 2010; 24: 27-40
- MUECK W, SCHWERS S, STAMPFUSS J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subject, specific patient populations and relevance of coagulation monitoring. Thrombosis Journal 2013; 11: 10
- NUTESCU EA, SPINLER SA, WITTKOWSKY A, et al. Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother 2009; 43:1064-83
- PERNOD G, ALBALADEJO P, GODIER A, et al. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor Xa inhibitors: proposals of the Working Group on Perioperative Haemostasis (GIHP) - March 2013. Arch Cardiovasc Dis 2013; 106:382-93
- SAMAMA MM, CONTANT G, SPIRO TE, et al. Laboratory assessment of rivaroxaban: a review. Thrombosis Journal 2013; 11:11
- SIÉ P, SAMAMA CM, GODIER A, et al. Surgeries and invasive procedures in patients treated long-term with an oral anti-IIa or anti-Xa direct anticoagulant. Proposals from the Perioperative Haemostasis Interest Group (GIHP) and the Haemostasis and Thrombosis Study Group (GEHT). Ann Fr Anesth Réanim 2011; 30: 645-50
- STUDT JD, ALBERIO L, ANGELILO-SCHERRER A, et al. Accuracy and consistency of anti-Xa activity measurements for determination of rivaroxaban plasma levels. J Thromb Haemost 2017; 15:1576-83
- VAN VEEN JJ, MAKRIS M. Management of perioperative anti-thrombotic therapy. Anaesthesia 2015; 70 (Suppl.1): 58-67
- WEITZ JI, QUINLAN DJ, EIKELBOOM JW. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation 2012; 126:2428-32