Challenging situations evolve quickly in an operating room. Classical coagulation tests needed to choose the most appropriate therapy often take too long (45-90 minutes) and depend on a haematology laboratory availability. On the other hand, conventional tests (TT, aPTT, TP, INR, etc) only reflect the initiation of the coagulation cascade in vitro and do not provide any information on platelet involvement, clot stability or fibrinolysis [28]. They are poor predictors of bleeding risk. The development of point-of-care tests (POC tests) has changed the situation, even if these tests are not as reliable as those performed in a dedicated laboratory. From a practical point of view, these tests can be split into 4 categories.
- Conventional tests of the in vitro coagulation cascade (PT, aPTT, TT);
- Activated clotting time (ACT);
- Clot viscoelastic strength tests (TEG™, ROTEM™, Sonoclot™);
- Platelet aggregability tests (Multiplate™, VerifyNow™, PFA-100™, etc).
The implementation of these tests in routine cardiac surgery aims to individualise and manage haemostasis in a directed manner instead of infusing various components indiscriminately. It proved to reduce postoperative bleeding rates, blood product consumption and morbidity compared to conventional management [27]. However, it requires adequate trained anaesthesia staff and continuous quality control.
Conventional tests
The usual coagulation tests are readily available, but are variously affected by different anticoagulants (see Monitoring and Table 8.4) [2,16].
- PT and INR are the test of choice for anti-vitamin K agents. PT is sensitive to direct anti-Xa agents (rivaroxaban, apixaban, edoxaban), but insensitive to direct anti-IIa agents (dabigatran). It is partially correlated with rivaroxaban plasma level, but less well with edoxaban level and very little with apixaban level. A normal PT does not exclude an anticoagulant effect of xabans.
- The aPTT performs well for assessing the effect of non-fractionated heparin (UFH), but is very approximate for low molecular weight heparins (LMWH). The aPTT is sensitive to dabigatran but non-linearly; it is non-sensitive to xabans. A normal aPTT does not exclude an anticoagulant effect of dabigatran.
- The TT (thrombin time) is hypersensitive to dabigatran; a normal TT excludes the presence of the substance. This over-sensitivity is corrected by the diluted TT (dTT) and calibrated for dabigatran (Hemoclot™), which allows a quantitative determination of its effect.
- Calibrated anti-Xa tests for the desired anticoagulant are required for quantitative determination of the effect of LMWH, fondaparinux and xabans.
ACT
The most commonly used test in cardiac surgery is the ACT (Activated clotting time): whole blood is brought into contact with an activator of the intrinsic pathway (celite, kaolin, glass or a combination of these), and heated to 37°C. The device measures the time required to transform the liquid blood into a coagulated gel. Results are prolonged in cases of deficiency of intrinsic pathway factors (factor XII, prekallikrein) or fibrinogen, and in the presence of aprotinin; the latter prolongs the ACT using celite as activator. It is therefore better practice to use a kaolin test when the patient is receiving aprotinin [6]. The ACT is mainly used to quantify the effect of heparin; although it is very sensitive, it is not very specific.
The normal ACT value is between 80 and 120 seconds. The minimum ACT necessary to avoid thrombotic or haemorrhagic problems during ECC is still debated, but a value of > 450 seconds, corresponding to a heparin level of > 2 U/mL, is generally accepted as a reference for standard circuits, and a value of > 250 seconds for pre-heparinised circuits. If the ACT is < 400 seconds (or < 250 seconds), it is recommended that ECC is not started without adding an additional dose of heparin (5,000 - 10,000 IU); which is rechecked after 3 minutes [13]. The ACT may not be sufficiently prolonged despite an adequate dose of heparin; it is probably due to heparin resistance related to AT III deficiency (see Intraoperative Coagulopathy). Although linear, the correlation of ACT with plasma heparin concentration is not exact: ACT tends to underestimate the actual heparin level. As a result, thrombin and fibrinolysis blockade may be less complete than expected [3].
Thromboelastography
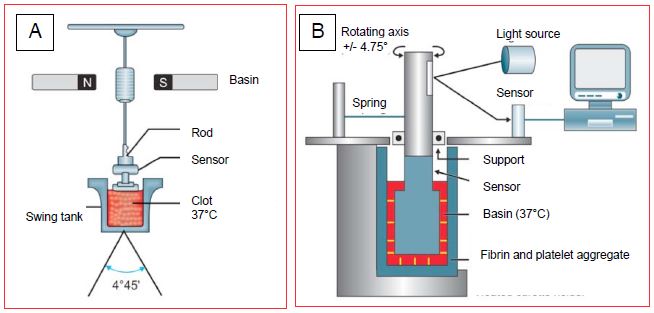
Thromboelastography, described by Hartert in 1948, is an elegant and rapid way of assessing the development and physical strength of the thrombus formed during the coagulation process, and its dissolution during fibrinolysis. The whole blood sample is placed in a small cuvette heated to 37°C; the development of fibrin is shown by a gradual coupling of rotational movements between the cuvette and a rod immersed in it (Figure 8.16).
Figure 8.16: Thromboelastography systems. A: TEG™ system; the progressive coupling of the movements of the vessel (oscillation of 4°45' around its axis) with those of a rod is recorded by an electromagnetic sensor; it reflects the formation and strength of fibrin bridges. B: ROTEM™ system; the oscillatory movements are imparted to the rod and not to the vessel and the transmission is optical and not electro-magnetic [from reference 25].
The electrical signal is displayed as a trace whose deflection is proportional to the strength of the clot (Figure 8.17).
This trace makes it possible to measure a series of parameters corresponding to the viscoelastic properties of the thrombus under a shear force corresponding to that prevailing in the venous circulation [25].
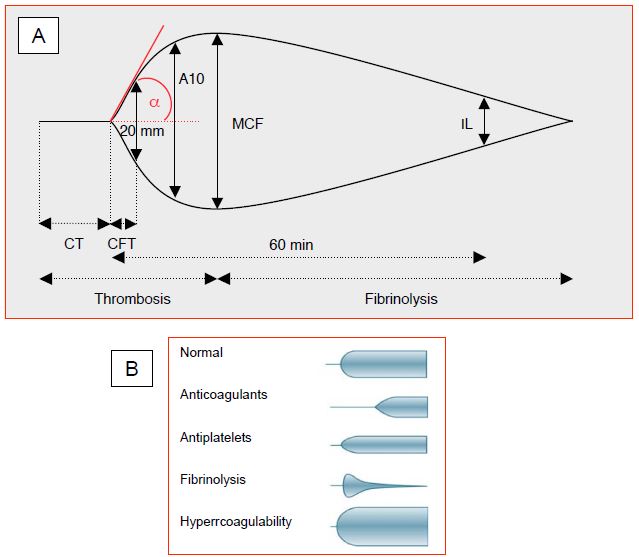
- CT (clotting time): time from the start of the measurement to the start of clot formation. Normal value: 55 seconds; the normal value varies according to the type of test.
- TTC (time to clot): time from the onset of clot formation to a curve amplitude of 20 mm. Normal value: 95 seconds; the normal value varies according to the type of test.
- Alpha angle (TTC slope): rate of fibrin formation. Normal value: 22-38° (native blood), 53-67° (kaolin activation).
- A10: amplitude 10 minutes after clotting time (CT); normal value: 43-68 mm. An amplitude of 0 mm means a complete absence of thrombus; the maximum of 100 mm indicates that the system is completely blocked by clot (beyond 10 minutes).
- MCF (maximum clot firmness, expressed in mm): maximum amplitude of the trace, usually at 15th minutes; it defines the final viscoelastic properties of the clot. Normal value: 47-71 mm.
- IL, LM (lysis index, or maximum lysis): percentage of clot firmness (MCF) at 30 or 60 minutes. Normal value: < 18% at 60 minutes.
Figure 8.17: Thromboelastogram. A: Schematic representation of a normal thromboelastogram. CT: clotting time. TFC: clot formation time (time from the start of clot formation to a curve amplitude of 20 mm). A10: amplitude 10 minutes after CT. MCF: maximum clot firmness. IL: fibrinolysis index. aA: Angle representing the slope of the TFC, measured from the point of clot initiation to an amplitude of 20 mm. B: Illustration of thromboelastograms corresponding to different pathologies [from reference 21].
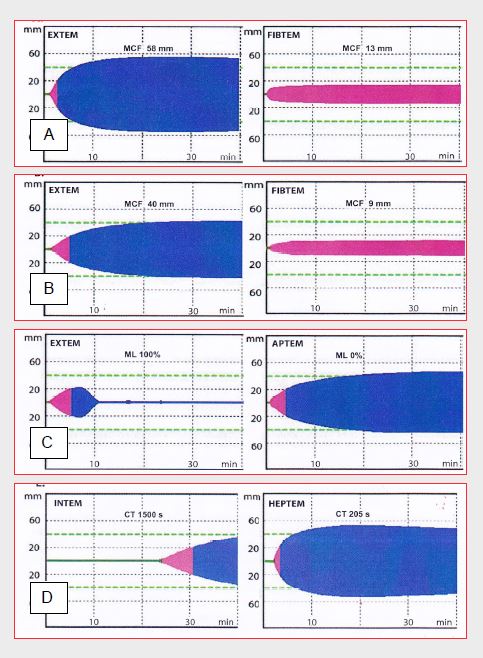
There are two models of thromboelastography available in bedside versions (POC-test): the thromboelastogram (TEG™) and the rotational thromboelastometer (ROTEM™). In the TEG™ system, blood is placed in a cuvette that oscillates on its 4°45' axis; fibrin development results in a progressive coupling of the movements of the tube with those of a rod, the oscillations of which are recorded by an electromagnetic sensor The ROTEM technology differs in that the oscillatory movements are imparted to the rod rather than the cuvette, and the transmission is optical rather than electro-magnetic (Figure 8.16). The system is more stable and easier to use. The sample consists of citrated and recalcified blood and is divided into aliquots with different activators (listed in brackets below) to allow several analyses to be performed in parallel. In addition to clotting time and clot kinetics, the ROTEM™ thus offers several other data (Figure 8.18) [24].
Figure 8.18: Examples of ROTEM™ thromboelastographic tracings. A: normal plot. B: thrombocytopenia plot. C: excessive fibrinolysis plot. D: plot under complete heparinisation (ECC). MCF: maximum clot firmness. CT: clotting time. ML: maximum lysis (percentage of MCF at 60 minutes) [from reference 24].
- INTEM (+ ellagic acid + phospholipid + calcium): information on the intrinsic pathway; INTEM TC is equivalent to aPTT.
- EXTEM (+ tissue factor + calcium): information on the extrinsic pathway, equivalent to PT.
- HEPTEM (ellagic acid + heparinase): neutralisation of heparin by heparinase; allows the diagnosis of abnormalities while the patient is on heparin (ECC).
- APTEM (tissue factor, calcium, + aprotinin): neutralisation of fibrinolysis by aprotinin; shows hyperfibrinolysis compared to EXTEM.
- FIBTEM (elimination of platelet participation in the clot by cytochalasin D): assessment of fibrinogen function, since thrombus formation is now solely dependent on fibrinogen.
- ECATEM (+ ecarin): information equivalent to the ecarin clotting time for the evaluation of direct thrombin inhibitors (bivalirudin, desirudin, dabigatran).
EXTEM, INTEM and APTEM are directly related to fibrinogen and platelet levels [26]. HEPTEM in combination with INTEM allows diagnosis of coagulation factor abnormalities while the patient is on heparin in ECC, thus allowing prediction of what needs to be corrected after ECC [22]. Prolongation of the INTEM CT (> 240 sec) and EXTEM CT (> 100 sec), as well as lengthening of the MCF and reduction of the alpha angle, are useful in determining the requirement for PCC (Prothrombin complex concentrate), which contains factors II, VII, IX, X [8]. However, excess heparin or protamine can also prolong INTEM-TC. A FIBTEM A10 value < 5 mm is a good indicator of hypofibrinogenemia (< 1 g/L); a FIBTEM of 10 mm corresponds to about 2 g/L fibrinogen (Clauss method); if the MCF of the EXTEM does not improve after fibrinogen infusion, platelet transfusion is indicated. Comparison of EXTEM and FIBTEM allows differential diagnosis between thrombocytopenia and hypofibrinogenemia, which offers the possibility of administering only the defective component. However, platelet function is not properly investigated by thromboelastography, except in a specific version of the TEG system (TEG-PlateletMapping™). The first results of these tests come within 5-10 minutes, but it takes up to 60 minutes for a full examination (depending on the duration of fibrinolysis). As these tests have a high negative predictive value, their normality in the presence of bleeding is a definite indication for surgical revision [16].
Platelet aggregability
Unfortunately, the thromboelastogram says nothing about platelet function. It is therefore advisable to add a platelet aggregability test, but platelet aggregability is a complex and multifactorial phenomenon, and it is difficult to judge its function with a standard, rapid and unambiguous test. Several tests are available but they assess different mechanisms of platelet function and have a modest degree of consistency. As a result, there is no clear consensus on the best method to use or on the value to be considered as the threshold beyond which the risk of bleeding becomes very high. Two devices are frequently used intraoperatively (see Chapter 29, Platelet activity tests) [4,5].
- Multiplate Electrode Aggregometry (MEA™). It is based on the increase in electrical impedance between electrodes immersed in plasma when they become covered with platelet clusters. It measures aggregation between thrombocytes through GP-IIb/IIIa receptors, the focal point of stimulation of all other platelet receptors. It assesses aggregation related to different agents depending on the agonist used: aspirin (arachidonic acid), ADP receptor blockers (ADP).
- VerifyNow™. This test measures the decrease in optical density when platelets form aggregates, facilitated by the presence of fibrinogen-coated microbeads. It assesses aggregation between thrombocytes via GP-IIb/IIIa receptors. Depending on the agonist used, it measures aggregation linked to different substances: arachidonic acid (aspirin) or ADP (clopidogrel, prasugrel, ticagrelor). The test is simple, fast, semi-automatic, based on the use of cartridges requiring no pipetting. It is performed at the patient's bed. It is only reliable within the normal limits of platelet count and haematocrit. It is relatively insensitive to very high and very low levels of platelet inhibition. The VerifyNow™ is currently the most widely used and best validated test in clinical trials.
These platelet aggregability tests performed prior to heparinisation would predict which patients will require the most red blood cell and platelet transfusions: an inhibition of >60% identifies 72-91% of polytransfused patients. They have a better predictive value than the time between surgery and last clopidogrel [22]. When their platelets are >70% inhibited, patients are 11 times more likely to transfuse, regardless of the duration of the interruption (area under the ROC curve for 70% threshold value: 0.77) [19]. However, apart from a few specific applications, none of the tests currently in use adequately detects hypo- or hyper-receptors to antiplatelets; none can individualise treatment to significantly improve clinical outcomes [11]. Since hypothermia-related platelet dysfunction (< 33°C) is reversible, testing should be performed on blood warmed to 37°C.
Targeted care
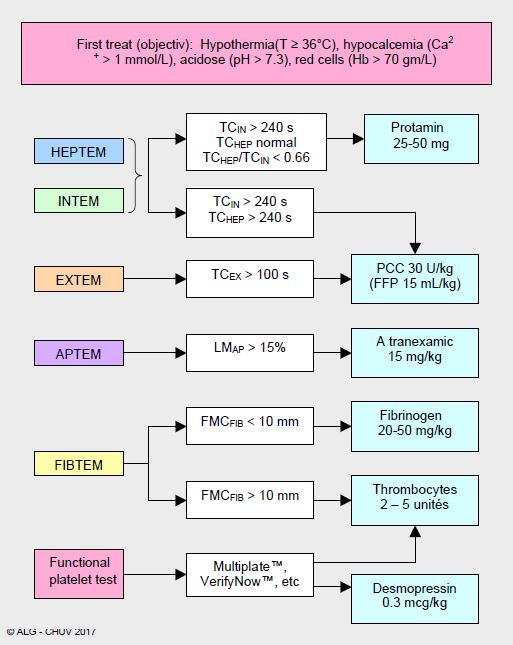
For a long time, haemostasis was performed in an empirical and indiscriminate manner, as there were no objective criteria or clinical evidence on which to base a rational attitude to the administration of procoagulants. The situation has changed significantly in the last decade with the development of specific coagulation tests that are easy to perform in the operating room. This has led to the development of logical goal-directed strategies, which avoid blindly injecting quantity of product not related to real need. An algorithm based on the use of thromboelastogram and a platelet aggregability test makes it possible to determine the origin of bleeding and to stratify the administration of the different haemostatic factors according to the deficiencies detected (example based on the ROTEM™ in Figure 8.19) [7,12,23].
Figure 8.19: Example of an algorithm for the administration of haemostatic agents based on the use of ROTEM™ at the end of ECT and supplemented by a platelet function test (CHUV, Lausanne) [12]. It is recommended to perform a new test after reversal of heparin with protamine. CT: clotting time (seconds). LM: maximum clot lysis at 60 min (%).MCF: maximum clot firmness (mm).
This approach is based on the selective use of the various elements involved in haemostasis, and not on the blind administration of the maximum number of components, which recalls the famous phrase of Marshal Ney (1769-1815): "The abundance of the firing compensates for the inaccuracy of the shot".
In its version for cardiac surgery, this algorithm varies between institutions, but in general consists of several identical steps (see Intraoperative haemotherapy) [1,7,12,27,28].
- Correction of physiological alterations: maintenance of pH > 7.3, temperature > 36°C, [Ca2+ ]i > 1 mmol/L, and Ht > 25%.
- Careful surgical haemostasis, compression, coagulation, tissue adhesives.
- Therapeutic measures taken based on results of gasometry, thromboelastograms and point-of-care platelet activity tests, supplemented by platelet and fibrinogen levels (laboratory). The first set of tests is performed during warming in ECC, before pump weaning, and treatment is started as soon as weaning is performed (infusion of clotting factors during ECC leads to filters thrombosis).
- If CTIN > 240 sec but CTHEP normal: protamine (0.8 mg per 1 mg heparin) for ACT < 130 sec.
- Administration of an antifibrinolytic (LMAP > 15%): tranexamic acid or ε-amino caproic acid (see Antifibrinolytics).
- If bleeding persists despite conventional measures:
- If CTIN and CTHEP > 240 sec + CTEX > 80 sec: fibrinogen (depending on plasma level), FFP (if hypovolemia).
- If CMFFIB < 9 mm: fibrinogen; maintain > 2.0 g/L with Fibrinogen™ 25-50 mg/kg; ensuring fibrinogen correction is the first step in correcting coagulation factors.
- If MCFFIB > 9 mm and thrombocytopenia (< 50,000/mcL): thrombocytes (5 U).
- If platelet aggregability is decreased (Multiplate™, VerifyNow™): desmopressin (0.3 mcg/kg), thrombocytes (2-5 U) (see Platelet Normalisation).
- If fibrin monomers + MCF < 9mm: factor XIII (Fibrogammin P® 10-30 IU/kg) [15].
- If factors II, VII, IX and X are reduced: prothrombin complex concentrate (PCC) with 3 or 4 factors (20-30 IU/kg) (see Coagulation factors).
- Extreme (rescue) measures, provided Ht (> 25%), fibrinogen (> 2.0 g/L), blood calcium (> 1 mmol/L) and platelets (> 70,000/mcL) are normalized, only justified in case of persistent bleeding despite use of all haemostatic means, including surgery, endoscopy and interventional radiology
- Activated PCC (FEIBA™, 50 IU/kg).
- rVIIa factor (NovoSeven™, 90 mcg/kg).
- A fall in Hb < 70 g/L requires blood bag transfusion; from 4 units onwards, it is routine to administer FFP simultaneously in a 1:1 ratio [7].
Several clinical studies have already demonstrated the benefits of this approach. The adoption of an algorithm based on thromboelastometry saves blood transfusions (RR 0.88), platelet transfusions (RR 0.78) and fresh frozen plasma (RR 0.68); it reduces the incidence of massive transfusions by 2.5 times; it halves the rate of surgical re-exploration for haemostasis and the rate of thrombo-embolic events. The length of stay in intensive care tends to decrease (-31 hours) [23]. On the other hand, it increases the use of fibrinogen and PCC (prothrombin complex concentrate) [9,10]. A first randomised controlled trial (100 cardiac surgery patients) comparing guidance by conventional tests to guidance by thromboelastography (ROTEM™) and platelet aggregometry (Multiplate™) clearly demonstrates that targeted monitoring decreases transfusions (5 versus 3 bags), administration of PCC (5 versus 0 units) and use of factor VIIa (12 versus 1 treated patients), but also morbidity (renal failure, sepsis, thrombosis) and mortality (20% versus 4%, p = 0.013) [27]. A second study corroborates these results: administration of blood products according to a ROTEM™-based algorithm significantly decreases the rate of erythrocyte (OR 0.50), platelet (OR 0.22) and PFC (OR 0.20) transfusions without changing fibrinogen infusions [14]. Life-saving measures such as massive transfusions, surgical re-exploration and Factor VIIa are less frequent, with no change in the complication rate. In high-risk bleeding situations such as cardiac surgery, polytrauma or liver transplantation, there is evidence that targeted coagulation management reduces overall costs [18].
Although thromboelastometry can better guide therapy in the event of bleeding, it has a low positive predictive value for bleeding risk in the postoperative setting. Its negative predictive value is high (89-94%), but it cannot determine which patients are likely to have high postoperative blood loss, in contrast to its good predictive capacity in polytrauma. Only the INTEM and HEPTEM clotting times show some correlation with bleeding risk (r = 0.67 and 0.66 respectively) [20]. The best predictors of bleeding remain duration of ECC, complexity of surgery and a protamine:heparin ratio > 1 [21]. The most important benefit of tests such as ROTEM is essentially to increase anaesthetists' knowledge of coagulation and thereby improve patient management.
| Intraoperative coagulation tests |
|
Tests performed in the operating room within < 20 minutes that allow the administration of specific haemostatic factors to be stratified according to the deficits detected instead of infusing various coagulation agents indiscriminately.
ACT: activated clotting time (normal 80 - 120 sec), used for monitoring heparinisation. Desired value :
- Conventional ECC > 450 sec
- Pre-heparinised circuit 250-300 sec
- Insufficient elongationafteran adequate dose of heparin: AT III deficiency
Thromboelastography (TEG™, ROTEM™): measurement of thrombus development and physical strength and subsequent dissolution during fibrinolysis.
- Clotting time
- Clot formation time
- Speed of fibrin formation
- Maximum clot firmness
- Maximum lysis of the clot
Allows differentiation of protamine, antifibrinolytic, fibrinogen, thrombocyte or coagulation factor requirements.
Platelet aggregability: assesses the residual effect of antiplatelet agents (fresh platelet transfusion, desmopressin).
The use of these tests within an algorithm based on targeted administration of procoagulants reduces the rate of postoperative bleeding, blood product consumption and morbidity compared to conventional management.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- BROWN C, JOSHI B, FARADAY N, et al. Emergency cardiac surgery in patients with acute coronary syndromes: a review of the evidence and perioperative implications of medical and mechanical therapeutics. Anesth Analg 2011; 112: 277-99
- CUKER A, SIEGAL DM, CROWTHER MA, et al. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 2014; 64:1128-39
- DESPOTIS GJ, JOIST JH, HOGUE CW, et al. More effective suppression of hemostatic system activation in patients undergoing cardiac surgery by heparin dosing based on heparin blood concentrations rather than ACT. Thromb Haemost 1996; 76: 902-8
- Favaloro EJ, LIPPI G, FRANCHINI M. Contemporary platelet function testing. Clin Chem Lab Med 2010; 48:579-98
- FERREIRO JL, SIBBING D, ANGIOLILLO DJ. Platelet function testing and risk of bleeding complications. Thromb Haemost 2010; 103:1128-35
- FINLEY A, GREENBERG C. Heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg 2013; 116:1210-22
- GHADIMI K, LEVY JH, WELSBY IJ. Perioperative management of the bleeding patient. Br J Anaesth 2016; 117(suppl 3):iii18-iii30
- GHADIMI K, LEVY JH, WELSBY IJ. Prothrombin complex concentrates for bleeding in the perioperative setting. Anesth Analg 2016; 122:1287-300
- GÖRLINGER K, DIRKMANN D, HANKE AA; et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decrease allogeneic blood transfusion in cardiovascular surgery. Anesthesiology 2011; 115: 1179-91
- GÖRLINGER K, FRIES D, DIRKMANN D, et al. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother 2012; 39:104-13
- GOROG DA, FUSTER V. Platelet function tests in clinical cardiology. Unfulfilled expectations. J Am Coll Cardiol 2013; 61:2115-29
- GRONCHI F, RANUCCI M. Perioperative coagulation in cardiovascular surgery. In: MARCUCCI C, SCHOETTKER P, editors. Perioperative hemostasis. Coagulation for anesthesiologists. Heidelberg: Springer Verlag, 2014, 243-66
- JOBES DR. Safety issues in heparin and protamin administration for extracorporeal circulation. J Cardiothorac Vasc Anesth 1998; 12:17-20
- KARKOUTI K, McCLUSKEY SA, CALLUM J, et al. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery. Anesthesiology 2015; 122:560-70
- KARKOUTI K, McCLUSKEY SA, CALLUM J, et al. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery. Anesthesiology 2015; 122:560-70
- KORTE WC, SZADKOWSKI C, GÄHLER A, et al. Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology 2009; 110: 239-45
- KOZEK-LANGENECKER SA. Perioperative coagulation monitoring. Best Pract Res Clin Anaesthesiol 2010; 24: 27-40
- KOZEK-LANGENECKER SA, AHMED AB, AFSHARI A, ALBALADEJO P, et al. Management of severe perioperative bleeding: Guidelines from the European Society of Anaesthesiology. First update 2016. Eur J Anaesthesiol 2017; 34: 332-95
- KWAK YL. KIM JC, CHOI YS, et al. Clopidogrel responsiveness regardless of the discontinuation date predicts increased blood loss and transfusion requirementafteroff-pump coronary artery bypass graTF surgery. J Am Coll Cardiol 2010; 56:1994-2002
- MEESTERS MI, BURTMAN D, VAN DE VEN PM, BOER C. Prediction of postoperative blood loss using thromboelastometry in adult cardiac surgery: cohort study and systematic review. J Cardiothorac Vasc Anesth 2018; 32:141-50
- MEESTERS MI, VEERHOEK D, DE LANGE F, et al. Effect of high or low protamine dosing on postoperative bleeding following heparin anticoagulation in cardiac surgery. Thromb Haemost 2016; 116:251-61
- RANUCCI M, BARYSHNIKOVA E, SORO G, et al. Multiple electrode whole-blood aggregometry and bleeding in cardiac surgery patients receiving thienopyridines. Ann Thorac Surg 2011; 91:123-30
- SERRAINO GF, MURPHY GJ. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: updated systematic review and meta-analysis. Br J Anaesth 2017; 118:823-33
- TANAKA KA, BOLLIGER D, VADIAMUDI R, NIMMO A. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J Cardiothorac Vasc Anesth 2012; 26:1083-93
- THAKUR M, AHMED A. A review of thromboelastography. Int J Periop Ultrasound Appl Technol 2012; 1: 25-9
- 26THEUSINGER OM, SCHRÖDER CM, EISMON J, et al. The influence of laboratory coagulation tests and clotting factor levels on Rotation Thromboelalstometry (ROTEM™) during major surgery with hemorrhage. Anesth Analg 2013; 117:314-21
- WEBER CF, GÖRLINGER K, MEININGER D. Point-of-care testing: a prospective randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012; 117: 531-47
- WEBER CF, KLAGES M, ZACHAROWSKI K. Perioperative coagulation management during cardiac surgery. Curr Opin Anaesthesiol 2013; 26: 60-84