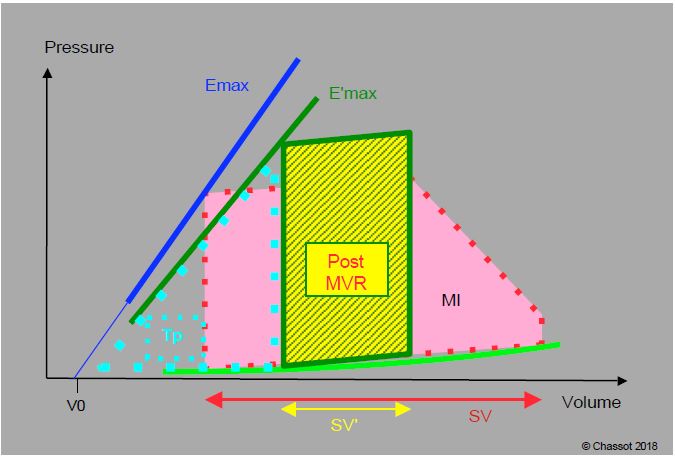
Figure 11.82: P/V loop of a patient with mitral regurgitation before (MI, red) and after mitral valve replacement (post-MVR, yellow and green). The prosthesis competence imposes an unknown isovolumetric contraction phase before ejection (vertical instead of oblique rise in systolic pressure gradient). Although the end-diastolic volume is increased, stroke volume and ejection fraction decrease, as indicated by the reduced surface area of the P/V loop. The slope of the maximum elastance (Emax) is lowered after ECC (E'max) because contractility is reduced. Compared to the situation before ECC, the work of pressure (area of the dotted triangle Tp) has increased, while the work of ejection (area of the green-yellow square) has decreased. For the LV, the energy yield is therefore less good and the situation very difficult: in addition to the functional handicap of ECC, there is the exchange of a volume overload (well tolerated) for a pressure overload (poorly tolerated) [according to ref 6].
Inotropic support is almost always required for patients coming off bypass [2]. This should be provided by non-alpha amines or inodilators: dobutamine, milrinone, levosimendant in refractory cases. The addition of an arterial vasodilator may occasionally be necessary if the SAR is too high. Enlarged ventricles require large filling volumes and sustained preload, especially in non-sinus rhythms. In the presence of pulmonary hypertension, there is a risk of right ventricular decompensation at the end of ECC. Positive pressure ventilation is beneficial because it reduces effective LV afterload. Weaning the patient from positive pressure ventilation can be a particularly delicate process and should not be considered too early, but only when left ventricular function has stabilised. Management of haemodynamics after correction of MI therefore requires :
- High preload,
- Low afterload,
- High contractility.
In this context, arterial hypotension should not be treated with vasopressors alone, as this will only increase LV difficulties. Alpha agents, which increase the SAR, are only indicated to ensure coronary, cerebral and renal perfusion pressure when this is compromised. In these circumstances, the most sensible approach is to place an intra-aortic balloon pump (IABP) to relieve the LV of the increased afterload while maintaining optimal perfusion pressure.
After mitral valve replacement with an attached bioprosthesis, one of the 3 tips of the prosthesis may partially obstruct the LVOT and induce abnormal flow acceleration. After mitral valve replacement, the LV pressure chamber may be subobstructed by the distal part of the anterior leaflet of the mitral valve (SAM, systolic anterior motion), leading to obstructive cardiomyopathy (HOCM effect) (see Mitral Valve Repair). In fact, the mitral coaptation zone moves closer to the LVOT when the mitral annulus is narrowed by a prosthetic ring, because the fixed point of the mitral annulus is at the mitro-aortic junction (fibrous trine), whereas the posterior part is thin and supported only by the ventricular musculature; it will therefore move anteriorly, causing an anterior displacement of the coaptation zone (Figures 11.56 and 11.57). Excessive anterior displacement (SAM) occurs in 4-11% of mitral valve repair cases [4,7]. It is associated with four different phenomena [4].
Video: Example of anterior displacement of the mitral leaflet (SAM) obstructing the left outflow tract in mesosystole in a case of severe hypertrophic cardiomyopathy (long-axis view of the LV 140°).
Video: Example of anterior displacement of the mitral leaflet (SAM) after mitral plasty (4-cavity 0° view); the anterior leaflet bends into the outflow tract in mesosystole; plasty of the posterior leaflet gives it a very altered appearance.
- The valvuloplasty ring is too restrictive; SAM is more common after insertion of a completely rigid and restrictive ring than after insertion of a semi-rigid and slightly restrictive open ring.
- The length of the posterior layer is excessive (excess tissue, insufficient resection).
- The ventricular cavity is too small: hypovolemia.
- The radial displacement of the posterior wall in telesystole is too great: over-stimulation β , vasoplegia.
The first two are related to the surgical procedure and occur in 5-15% of mitral valve repair cases [3]. They are related to haemodynamics and can be corrected by treating the dynamic obstruction of the LVOT [4,7]:
- Increase preload (filling);
- Increase in afterload by vasoconstrictors (neosynephrine, noradrenaline);
- Decreased contractility (withdrawal of catecholamines, β-blockade with esmolol);
- Re-operation: rarely required (6-8% of cases) [1,7].
| MI surgery: post-ECC |
|
Correction of mitral regurgitation removes the LV pressure valve. This exchanges volume overload at low resistance (well tolerated) for pressure overload (SAR) at normal volume (poorly tolerated). Isovolumetric contraction is restored (increase in pressure work). For the LV, the situation after ECC is therefore more difficult than before correction. Support : Plasty surgery is functionally better tolerated than replacement with a prosthesis |
References
- CRESCENZI G, LANDONI G, ZANGRILLO A, et al. Management and decision-making strategy for systolic anterior motion after mitral valve repair. J Thorac Cardiovasc Surg 2009; 137:320-5
- GERHARDT MA, BOOTH JV, CHESNUT LC, et al. Acute myocardial beta-adrenergic receptor dysfunction after cardiopulmonary bypass in patients with cardiac valve disease. Circulation 1998; 98:II275-II281
- GILLINOV AM, COSGROVE DM, LYTLE BW, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg 1997; 113:467-73
- LOULMET DF, YAFFEE DW, URSOMANNO PA, et al. Systolic anterior motion of the mitral valve: a 30-year perspective. J Thorac Cardiovasc Surg 2014; 148:2787-94
- MELISURGO G, AJELLO S, PAPPALARDO F, et al. Afterload mismatch after MitraClip insertion for functional mitral regurgitation. Am J Cardiol 2014; 113:1844-50
- ROSS J. After-load mismatch in aortic and mitral valve diases: Implications for surgical therapy. J Am Coll Cardiol 1985; 5:811-5
- VARGHESE R, ANYANWU AC, ITAGAKI S, et al. Management of systolic anterior motion after mitral valve repair: an algorithm. J Thorac Cardiovasc Surg 2012; 143:S2-7
- WONG CYH. SPOTNITZ HM. Systolic and diastolic properties of the human left ventricle during valve replacement for chronic mitral regurgitation. Am J Cardiol 1981; 47:40-5.