Aortic insufficiency is often associated with stenosis because the calcified valve can no longer seal. Although it has no haemodynamic significance as long as the heart is beating, AI may be sufficient to fill and dilate the LV during cardioplegia at the start of ECC. The ventricle must therefore always be carefully monitored.
Ventricular hypertrophy makes adequate preservation by cardioplegia difficult, particularly in the presence of associated coronary artery disease. The usual technique of perfusing the aortic root may be supplemented by retrograde cardioplegia via the coronary sinus or replaced by direct cannulation of the coronary ostia (Spencer cannulae) after opening the aorta. As a result of these difficulties in preservation, the LV may fail on weaning off pump, even if its function was satisfactory preoperatively. At the time of weaning, a hypertrophied ischaemic heart may contract and become dysfunctional if calcium is administered (stone heart). The extent and significance of this phenomenon is still widely debated.
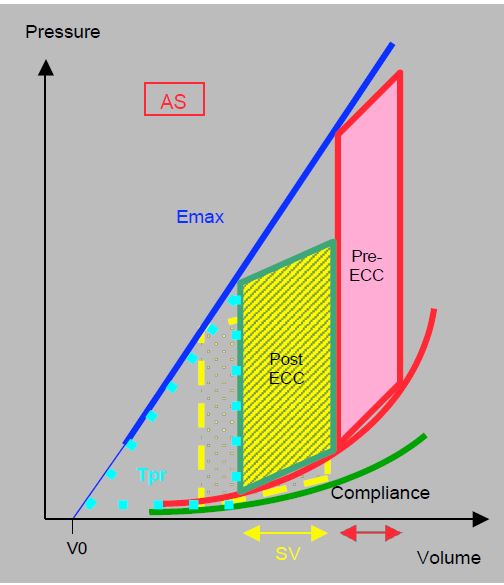
If the cardioplegia has been satisfactory and the aortic clamping time relatively short, myocardial performance improves considerably as soon as the ejection obstruction is removed, because wall stress normalises almost immediately. The transvalvular gradient is reduced as the useful surface area of prosthetic valves is in the order of 1.3 - 2.5 cm2 . For the same ejection work as before AVR, the LV ejects a greater systolic volume at a lower pressure regime (Figure 11.120). The work of pressure, the main energy consumer, is much lower than before valve replacement. The ratio of work of pressure to work of ejection (Tpr/Tej) improves considerably and the mechanical efficiency of the ventricle is increased: it consumes less O2 for the same systolic performance. Although its diastolic function remains unchanged, the rate of myocardial shortening in systole (strain rate) is increased by 40% and its wall stress is reduced by 50% [1]. This immediate improvement is the reason why the indications for AVR in cases of stenosis can be very broad, even in elderly patients or those in poor general condition.
Figure 11.120: Schematic representation of the effect of lifting the ejection stenosis during AVR using the pressure/volume (P/V) loop. The surface area of the loop before and after bypass is identical. For the same ejection work, represented by the surface area of the P/V loop, the LV ejects a greater systolic volume (SV) at a lower pressure regime. The work of pressure, represented by the area of the Tpr triangle, is much less than before the valve replacement. For the LV, the improvement is immediate.
The situation is different for the right ventricle. It has undergone the stresses of cardioplegia, arrest and reperfusion without any improvement in its haemodynamic conditions, as it does not benefit directly from the elimination of the aortic stenosis. On the contrary, when the patient is off pump, there are significant variations in preload and a certain increase in afterload (mechanical ventilation, closure of the pericardium and sternum, pulmonary hypertension). Its longitudinal systolic shortening (tricuspid annulus prolapse, strain) is reduced [1]. Its failure is associated with a 5- to 25-fold increase in cardiovascular morbidity and mortality [2].
Any mean gradient between the LV and the aorta greater than 20 mmHg should raise the suspicion of prosthesis malfunction or prosthesis-patient mismatch.
- Blocked winglet: the winglet remains motionless, usually in an intermediate position, with only one opening for blood flow.
- Prosthesis size too small for the patient's height: S < 0.85 cm2/m2 (see Heart valve prostheses, Patient-prosthesis mismatch).
However, several phenomena can lead to a falsely high transprosthetic gradient [3].
- Transient episode of high stroke volume: hypervolaemia, too rapid transfusion from ECC.
- Dynamic subaortic stenosis (HOCM effect): the removal of the obstacle to ejection can cause a hypertrophied ventricle to collapse in systole, because its cavity is naturally small and the outflow chamber is very muscular; the phenomenon is similar to obstructive cardiomyopathy (HOCM).
Video: Transgastric long-axis view of a St Jude aortic prosthesis with both wings functioning normally; the patient has severe concentric hypertrophy of the LV with very thick walls.
This constriction of the outflow tract in systole creates a dynamic obstruction which may represent a gradient of 25-40 mmHg (see Dynamic subaortic stenosis below). This gradient must be subtracted from the total transaortic gradient measured on the Doppler echo to obtain the prosthesis' own gradient (Figure 11.121).
Figure 11.121: Calculation of pressure gradient after AVR. With continuous Doppler we have a double envelope to measure Vmax in the LVOT and in the LVOT + prosthesis assembly. Using the simplified Bernoulli equation (ΔP = 4 (Vmax)2), we find a gradient of 22 mmHg. If we use the equation that takes into account the Vmax in the LVOT, i.e.ΔP = 4 (V2 - V1)2, the gradient across the prosthesis is only 10 mmHg. In the case of an unmounted bioprosthesis, this difference has a direct surgical impact, as 22 mmHg is an excessive gradient for this type of valve and may lead to a return to bypass surgery that is not justified.
- Even in the absence of muscular stenosis, the velocity in the LV outflow tract may exceed 1.5 m/s due to significant sympathetic stimulation and transient hypervolemia. In this case, Bernoulli's equation must be corrected to take account of the Vmax in the LVOT : ΔP = 4 (V2 - V1)2, where V2 is the Vmax through the prosthesis and V1 is the Vmax in the LVOT. The gradient generated in the LVOT is then subtracted from the total gradient to give the prosthesis specific gradient.
- Pressure recovery: kinetic energy is converted back into pressure beyond the zone of maximum acceleration where pressure is minimal; as echocardiography measures pressure precisely at maximum velocity, it tends to overestimate the true gradient (see Figure 11.116).
- The presence of intra-aortic balloon pump (IABP) significantly reduces the pressure in the ascending aorta at the beginning of systole as the balloon deflates; this results in an exaggerated gradient between the LV and the aorta; the gradient should be measured during a momentary cessation of IABP. Significant dilatation of the ascending aorta has the same effect, but to a lesser extent.
Provided that preoperative function is preserved, the immediate postoperative course is uncomplicated, even in elderly patients; extubation is early. Inotropic support is not always necessary after simple AVR because of the immediate improvement in LV function. If it is required, the inotropic infusion can usually be stopped quickly. Air or valve debris can easily get into the coronary ostia, causing a picture of truncal ischaemia with ST segment elevation at the end of the bypass. Neurological deficits are always possible due to embolization of calcified and friable fragments during aortotomy and valve resection. The postoperative stroke rate is 3-6%, which is 2-3 times higher than the average for cardiac surgery.
The proximity of the AV node to the aortic annulus at the level of the septum can lead to iatrogenic complete AV block after prosthesis implantation. If the patient's rhythm has to be paced due to bradycardia or block, it is preferable to pacing in the atrioventricular (A-V) mode to benefit from the contribution of atrial contraction, as LVH regresses slowly and diastolic dysfunction persists postoperatively. This maintains abnormally high filling pressures relative to LV volume.
The occurrence of MI after AVR can be related to several different phenomena.
- Re-opening of the mitral valve in mid-systole in dynamic subaortic stenosis (SAM); although significant, this MI is brief and does not last throughout systole; it is meso-tesystolic. It is often a warning sign of the HOCM effect (see below).
- Retraction of the anterior leaflet by prosthesis anchoring points; the proximity of the aortic and mitral rings at the trine means that slightly wide points in this area exert traction on the anterior leaflet and may prevent it from occluding properly.
Video: Proximity of the mitral valve and the aortic prosthesis in a short-axis transgastric view of the mitral valve; the St-Jude aortic prosthesis is located at the bottom left of the screen image; the movement of the two wings during the cardiac cycle can be seen.
- Perforation at the base of the anterior leaflet in decalcification of the annulus and calcific flow on the mitral valve.
- LV dysfunction; MI is a common associated sign of acute LV dilatation or ischaemia.
- Presence of MI prior to ECC, whether organic (calcific disease, degeneration, age-related changes) or functional (mitral annular calcification, LV dysfunction or dilatation). In most cases, removal of the obstruction reduces LV wall stress to such an extent that MI is less significant than preoperatively.
If the size of the prosthesis is appropriate for the patient, LVH will regress in the first 3 months, but the LV will not fully recover from the remodelling imposed by the stenosis.
| Surgery for aortic stenosis: after ECC |
| AVR removes the obstruction to ejection, immediately relieving pressure on the LV and significantly improving its function. The patient is usually discharged from bypass without difficulty, except in the case of pre-operative ventricular dysfunction, associated ischaemia or cardioplegia problems. The functional prognosis of AVR is excellent, even in the elderly. However, diastolic dysfunction due to LVH persists postoperatively (high filling pressures).
However, a number of problems may arise. -Excessive gradient (ΔPmean > 20 mmHg): insufficient prosthesis size (S < 0.85 cm2/m2) Blocked winglet
- HOCM effect: increases preload, vasoconstrictive, reduces β catecholamines, esmolol
- New MI: dysfunction, traction on anterior lamina, SAM
- Paravalvular leak: return to pump if large
- LV dysfunction: lack of cardioplegia, ischaemia
- AV block
|
References
- DUNCAN AE, SARWAR S, KASHY BK, et al. Early left and right ventricular response to aortic valve replacement. Anesth Analg 2017; 124:406-18
- HADDAD F, DENAULT AY, COUTURE P, et al. Right ventricular myocardial performance index predicts perioperative mortality or circulatory failure in high-risk valvular surgery. J Am Soc Echopcardiogr 2007; 20:1065-72
- RAJANI R, MUKHERJEE D, CHAMBERS JB. Doppler echocardiography in normally-functioning replacement aortic valve: a review of 129 studies. J Hesart Valve Dis 2007; 16:519-35
- ZOGHBI WA, ADAMS D, BONOW RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the ASE developed in collaboration with the SCMR. J Am Soc Echocardiogr 2017; 30:303-71