Blood transfusion is main topic of a separate chapter (see Chapter 28) and will be discussed here briefly: overall patient blood management, risks of transfusion and recommendations in cardiac surgery.
Comprehensive blood savings management
Blood transfusion is only one component of an integrated blood-saving strategy that considers the patient's blood as an irreplaceable asset deserving maximum protection. It is based on five principles [5,9,25].
- Correction of preoperative anaemia and/or coagulopathy ;
- Saving the patient's blood cells and coagulation factors, limiting blood loss;
- Rational administration of blood products, restrictive transfusion thresholds ;
- Use of algorithms based on laboratory tests performed in the operating room;
- Improvement of tissue DO2 by hemodynamic and ventilatory optimization.
This strategy includes measures that are listed in Table 8.9 and detailed in Chapter 28 (Comprehensive Blood Management Strategy) [2,8].
Preoperative anaemia deserves special mention because it is as easy to correct as it is dangerous for survival. Moderate anaemia (100-120 g/L) is associated with 40% increased morbidity [25] and 16% increased mortality [13]. It is present in about 30% of cases [13]. Preoperative treatment is straightforward, as most cases are due to iron deficiency anaemia: iron, folic acid, vitamin B12, possibly EPO; transfusion is not a consistent option, as a single blood bag increases perioperative risk by 2-3 times [24]. In addition to this baseline anaemia, there is intraoperative blood loss and blood loss from laboratory tests, which averages 450 mL in cardiac surgery and 1,000 mL for 2 weeks in intensive care [15].
The purpose of transfusion is to improve O2 transport to the tissues (DO2 ), not to correct haemoglobin (Hb) levels. It is based on haemodynamic imbalance, ischaemic organ dysfunction and the patient's cardiopulmonary reserve. However, Hb is a practical benchmark that is still widely used and allows simple criteria to be defined. Provided the patient is normovolaemic and the source of bleeding is controlled, transfusion is indicated for the following Hb values [1,2,3,4,12].
- In a healthy individual with normal haemodynamics, transfusion is indicated if the Hb is < 60 g/L.
- Perioperatively, a Hb value of 70-80 g/L is a reasonable threshold for transfusion.
- In at-risk populations (coronary ischaemia, ventricular failure, stroke, nephropathy, advanced age), the transfusion threshold may be raised to 80-90 g/L; the same applies to febrile, septic or ARDS patients.
- Transfusion is unlikely to improve DO2 when Hb is ≥ 100 g/L, except in cyanogenic heart disease (R-L shunt, pulmonary hypertension) or severe ARDS.
One blood bag increases Hb level by about 1 g/L and the Ht by 3% in an adult. Red blood cell transfusion affects coagulation. It improves platelet function by increasing thromboxane production and ADP release, and increases thrombin production [20]. In addition, the marginalisation of platelets in blood stream by mass of erythrocytes that remain in the middle of the stream increases the chances of thrombocyte attachment to the vessel wall [22].
Transfusion-related risks
About 1-4% of transfused patients develop complications. Risks of blood product transfusion can be grouped under several headings (see Chapter 28 Risks) [4].
- Transfusion reaction: allergy, febrile reaction, circulating volume overload (1-5%).
- Reaction related to blood bag components (hyperkalaemia, hypocalcaemia, etc).
- Immunomodulation (leukocyte-related reactions): Transfusion-Related Acute Lung Injury (TRALI), cancer recurrence, inflammatory reaction, graft-versus-host disease (1:104 ).
- ABO haemolytic reaction (1:105 ).
- Transmission of bacterial, viral or prion-related diseases (1:106 ).
- Economic risk (cost, supply difficulties).
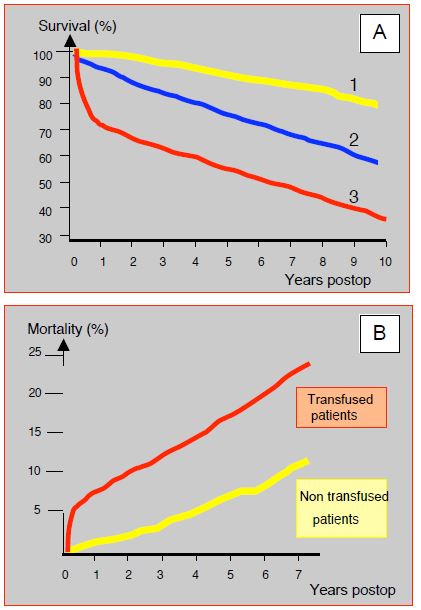
Transfusion is directly related to postoperative morbidity and mortality after cardiac surgery. A comparative analysis of patients survival who receive 1-2 blood bags compared to those who are not transfused in a group of 3,254 cases shows an overall increase in mortality of 16% in transfusion recipients; death risk is multiplied 1.7 times (HR 1.67) at 6 months, but the difference is no longer significant at 5 years (HR 1.06) [21]. However, the "collateral damage" of transfusion seems to be present in the long term. In a series of 1,915 cardiac surgery patients followed up at 5 years, transfusion (34% of the group) resulted in a 70% increase in mortality (HR 1.7) in the long term, even after adjustment for comorbidities and associated factors; in multivariate analysis, transfusion remained an independent determinant of mortality [7]. A 10-year follow-up study of 10,289 CABG patients showed a significant reduction in survival of transfused patients that was proportional to the number of blood bags received (Figure 8.22A) [14]. In this study, the long-term risk of 3 blood bags is equivalent to that of reoperation, common trunk disease or low ejection fraction; the risk of 6 units of blood is the same as that of renal failure or ventricular failure. An analysis of the 7-year outcome of 8,724 patients showed an odds ratio of 3.38 and 3.35 respectively for infections and ischaemic complications (MI, stroke, renal failure) in transfused patients compared to non-transfused patients (Figure 8.22B) [18]. However, these observations mainly concern polytransfused patients; indeed, the administration of 1-2 bags of leukoreduced blood does not seem to affect long-term vital prognosis [16].
Figure 8.22: Effect of transfusions on postoperative mortality in cardiac surgery. A: Reduction in 10-year survival of transfused patients in proportion to the number of blood bags received during coronary artery ECC [12]. 1: No transfusion (yellow line). 2: transfusion of 3 units (blue line). 3: transfusion of 6 or more units (red line). B: Increased mortalityaftercardiac surgery in transfused patients compared to non-transfused patients. The difference occurs mainly in the first six months, but the curves continue to diverge slightly in the long term [18].
Recent comparisons between restrictive (Hb < 70 g/L) and liberal (Hb > 90 g/L) transfusion thresholds show no difference in short-term or long-term mortality, which means that the restrictive attitude is safe [4]. In cardiac surgery, however, the situation seems different. The restrictive strategy tends to increase cardiovascular mortality (OR 1.64), although it does not alter rate of complications or morbidity [17]; in non-surgical acute coronary syndrome, it increases risk morbidity (OR 1.78) [6]. Cardiac surgery patients are therefore a fragile population that deserves a transfusion threshold of 80-90 g/L Hb rather than a low threshold (Hb 70 g/L) appropriate for a non-cardiovascular population. The apparent discrepancy between the latter two studies and those cited above is that, for the same Hb level, transfused patients have a poorer prognosis than those who were not transfused. In other words, acute anaemia is dangerous, and transfusion to correct it makes the situation worse. The conclusion is to avoid preoperative anaemia at all costs and to keep intraoperative blood loss to a minimum.
Recommendations for cardiac surgery
A few simple measures can significantly reduce the consumption of blood products and change patients prognosis [19].
- Treat preoperative anaemia. Too many patients arrive in the operating room with an Hb of 100-110 g/L. An Hb level of < 120 g/L in women and < 130 g/L in men is responsible for increased perioperative morbidity and mortality [23], and this risk is doubled in case of transfusion [10,11]. Treatment with iron, vitamin B12, folic acid and, if necessary, erythropoietin (EPO) can normalise the values preoperatively and allow surgery to be performed with a better reserve.
- Restrict transfusions: restrictive threshold (Hb < 80 g/L, Hb < 90 g/L in the elderly or at risk).
- Limit blood loss: rigorous haemostasis, normovolaemic pre-ECC haemodilution, blood recovery, ultrafiltration.
- Avoid intraoperative coagulopathy: normothermia, acid-base balance, calcium, antifibrinolytics. Routine use of thromboelastography to assess coagulation factor deficiencies and selectively compensate for them: protamine, tranexamic acid, fibrinogen, PCC, factor XIII.
- Improve tolerance to anaemia: FiO2 0.8-1.0, muscle relaxation, deep anaesthesia, normovolaemia.
| Blood transfusion |
|
Red blood cell transfusion aims at improving tissue DO2 . Indications (normovolaemic patients and controlled bleeding):
- Transfusion is indicated if Hb is < 60 g/L
- Perioperatively, transfusion is usually indicated if the Hb is < 70-80 g/L
- In populations at risk (coronary ischemia, ventricular failure, stroke, nephropathy, advanced age), the threshold for transfusion can be raised to 80-90 g/L
Transfusion is unlikely to improve DO2 when Hb is ≥ 100 g/L, except in cyanotic heart disease (R-L shunt, pulmonary hypertension) or ARDS.
Transfusion increases the risk of infection, ARDS and renal failure. Like anaemia, it increases postoperative morbidity and mortality by about 15% (relative). Acute anaemia is dangerous, and transfusion to correct it makes the situation worse. It is important to avoid preoperative anaemia and to limit intraoperative blood loss.
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- APFELBAUM JL, NUTALL GA, CONNIS RT, et al. Practice guidelines for perioperative blood management. An updatesd report of the ASA task force on perioperative blood management. Anesthesiology 2015; 122:241-75
- BOER C, MEESTERS MI, MILOJEVIC M, et al. 2017 EACTS/EACTA Guidelines on patient blood management for adult cardiac surgery. J Cardiothorac Vasc Anesth 2018; 32:88-120
- CARSON JL, GUYATT G, HEDDLE NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016; 316:2025-35
- CARSON JL, TRIULZI DJ, NESS PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377:1261-72
- CLEVENGER B, MALLETT SV, KLEIN AA, RICHARDS T. Patient blood management to reduce surgical risk. Br J Surg 2015; 102:1325-37
- DOCHERTY AB, O'DONNELL R, BRUNSKILL S, et al. Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non-cardiac surgical setting: systematic review and meta-analysis. BMJ 2016; 352:i1351
- ENGOREN MC, HABIB RH, ZACHARIAS A, et al. Effect of blood transfusion on long-term survivalaftercardiac operation. Ann Thorac Surg 2002; 74:1180-6
- FERRARIS VA, BROWN JR, DESPOTIS GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011; 91:944-82
- GOODNOUGH LT, SHANDER A. Patient blood management. Anesthesiology 2012; 116: 1367-76
- HABIB RH, ZACHARIAS A, SCHWANN TA, et al. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg 2003; 125:1438-50
- HABIB RH, ZACHARIAS A, SCHWANN TA, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injuryaftercoronary revascularization: Implications on operative outcome. Crit Care Med 2005; 33:1749-56
- KLEIN AA, ARNOLD P, BINGHAM RM, et al. AAGBI Guidelines: the use of blood components and their alternatives. Anaesthesia 2016; 71:829-42
- KLEIN AA, COLLIER TJ, BRAR MS; et al. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia 2016; 71:627-35
- KOCH CG, LI L, DUNCAN AI, et al. Transfusion in coronary artery bypass graTFing is associated with reduced long-term survival. Ann Thorac Surg 2006; 81:1650-7
- KOCH CG, REINEKS EZ, TANG AS, et al. Contemporary bloodletting in cardiac surgical care. Ann Thorac Surg 2015: 99: 779-84
- KOSTER A, ZITTERMANN A, BÖRGERMANN J, et al. No significant association between the transfusion of small volumes of leukocyte-depleted red blood cells amd mortality over 7 years of follow-up in patients undergoing cardiac surgery: a propensity score matched analysis. Anesth Analg 2018; 126:1469-75
- MURPHY GJ, PIKE K, ROGERS CA, et al. Liberal or restrictive transfusionaftercardiac surgery. N Engl J Med 2015; 372:997-1008
- MURPHY GJ, REEVES BC, ROGERS CA, et al. Increased mortality, postoperative morbidity, and costafterred blood cell transfusion in patients having cardiac surgery. Circulation 2007; 116:2544-52
- NALLA BP, FREEDMAN J, HARE GMT, MAZER CD. Update on blood conservation for cardiac surgery. J Cardiothorac Vasc Anesth 2012; 26:117-33
- PEYROU V, LORMEAU JC, HERAULT JP, et al. Contribution of erythrocytes to thrombin generation in whole blood. Thromb Haemost 1999; 81: 400-6
- SURGENOR SD, KRAMER RS, OLMSTEAD EM, et al. The association of perioperative red blood cell transfusions and decreased long-term survivalaftercardiac surgery. Anesth Analg 2009; 108:1741-6
- UIJTTEWAAL WS, NIJHOF EJ, BRONKHORST PJ, et al. Near-wall excess of platelets induced by lateral migration of erythrocytes in flowing blood. Am J Physiol 1993; 264: H1239-44
- 23VAN STRATEN AH, HAMAD MA, VAN ZUNDERT AJ, et al. Preoperative hemoglobin level as a predictor of survivalaftercoronary artery bypass graTFing: a comparison with the matched general population. Circulation 2009; 120:118-25
- WHITLOCK EL, KIM H, AUERBACH AD. Harms associated with single unit perioperative transfusion: retrospective population-based analysis. BMJ 2015; 350: h3037
- ZACHAROWSKI K, SPAHN DR. Patient blood management equals patient safety. Best Pract Res Clin Anaesthesiol 2016; 30:159-69