- AI decreases when APdiast decreases;
- LV ejection is improved when APsyst decreases.
Because it is both a volume and pressure overload, AI is less well tolerated than MI [4]. The amount of blood that regurgitates from the aorta to the LV depends on
- The surface area of the orifice kept open in diastole (severe AI: > 0.3 cm2).
- Duration of diastole: bradycardia increases the duration of diastole and therefore the volume of regurgitation.
- The pressure gradient between the aorta and the LV in diastole: this depends on the pressure in the ascending aorta, the SAR and ventricular compliance; it is highest in protodiastole and decreases with increasing AI (rapid equilibration of aortic and intraventricular pressure).
Tachycardia shortens diastole and the volume regurgitated per cardiac cycle decreases. As a result, LV dilation is slowed. Bradycardia is a major hazard because of the overfilling of the LV during long diastole, when the AI pours a large amount of blood into the ventricle. The optimum rate is probably around 80-90 beats per minute. It is certainly dangerous to go below 60 beats per minute.
Chronic aortic insufficiency
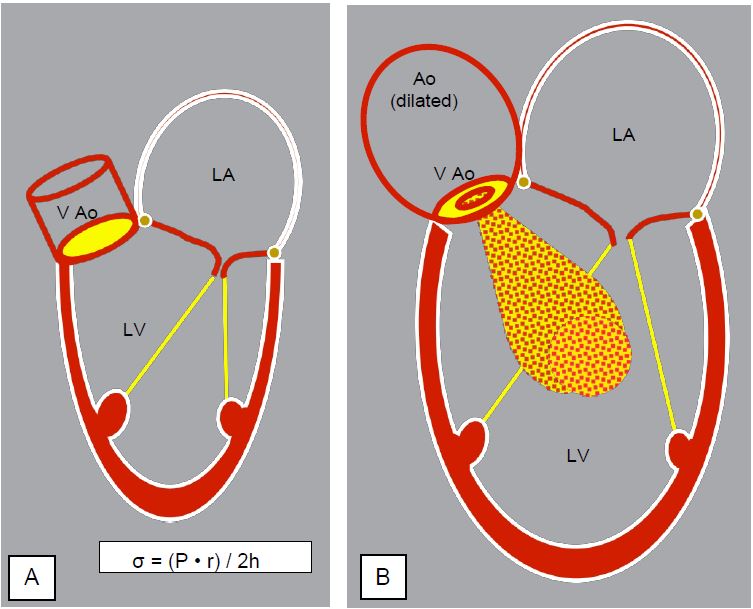
As ventricular dilatation increases wall stress (σ ), the LV will thicken and hypertrophy to keep this stress normal (Laplace's law: σ = (P · r) / 2h). This leads to eccentric or dilatory hypertrophy by serial replication of the sarcomeres and elongation of the fibres (Figure 11.128) [9].
Over time, myocardial fibrosis progresses; it is clearly visible on MRI and is doubly punishing: fibrotic myocardium no longer participates in contraction but reduces diastolic compliance. Laplace's law states that the larger the ventricle, the greater the wall stress required to develop the same systolic pressure. Chronic AI causes the greatest ventricular enlargement of all cardiopathies: ventricular mass can be impressive, reaching > 600 g (bovine heart). With regurgitation rates of up to 5-8 L/min, the LV is forced to deliver 10-12 L/min to maintain peripheral perfusion ! Exercise is relatively well tolerated because the SAR decreases and tachycardia shortens diastole: the regurgitant fraction decreases [7]. The usual indices of systolic function, such as ejection fraction, are generally within normal limits, but do not reflect the true contractility of the myocardium with maximum use of preload reserve. The telesystolic dimension of the LV (diameter > 2.5 cm/m2 ) is a more useful index for determining the patient's prognosis, as it is less dependent on preload [3]. The ratio of wall stress to telesystolic volume is the best predictor of functional recovery after AVR [10].
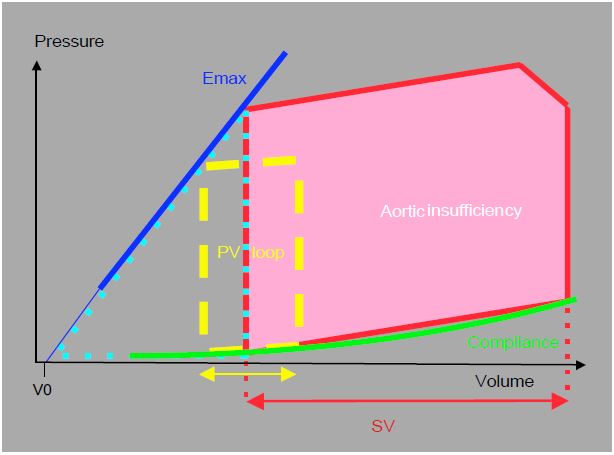
As long as systolic function is preserved, compliance is preserved; the dilated ventricle accommodates large volumes at still normal filling pressures; progressive volume overload has shifted the entire compliance curve to the right (Figure 11.129). However, the slope of the diastolic P/V relationship is straightened at high volumes; in acute failure the LV operates on the rising part of the curve, i.e. at high diastolic pressure. The pressure-volume diagram for AI also shows that the Emax-compliance-P/V loop triangle, the area of which represents the internal work of pressure (IWP), is of variable size. During the period of compensation, the ventricle expends more energy on the external work of ejection; this ratio is favourable for energy efficiency and O2 consumption . However, as dilation worsens, wall tension becomes excessive and the effective afterload of the LV increases; the IWP increases.
- Increased O2 demand due to high wall tension;
- Decreased O2 supply due to low aortic diastolic pressure and high intraventricular pressure (coronary perfusion pressure: CPP = PAdiast - PtdLV);
- Contractile mass increases disproportionately to the development of the coronary capillary network.
| Pathophysiology of aortic regurgitation |
|
AI increases LV Vtd; in diastole the ventricle is filled by the diastolic pressure of the aorta (40-80 mmHg). Wall stress is increased in the protocole, which represents an increase in effective afterload. AI is a volume and pressure overload. It induces large ventricular dilatation.
AI increases as SAR increases and decreases as SAR decreases. LV ejection is facilitated when its afterload is low because its dilation makes it very sensitive to systolic wall stress. The decrease in SAR is therefore doubly important:
- AI decreases when diastolic pressure falls.
- LV ejection is improved when systolic pressure falls.
The longer the diastole, the greater the regurgitant volume; tachycardia allows it to be broken down into smaller units, thereby reducing the regurgitant fraction and ventricular dilatation. Tachycardia is beneficial, whereas bradycardia is dangerous. Optimum rate: 80-90 beats/min.
AI induces eccentric hypertrophy. EF does not reflect true LV function; telesystolic dimensions are a better criterion. Function is reduced when Vts > 2.5 cm/m2.
Myocardial ischaemia develops late due to three phenomena:
- ↑ mVO2 on ↑ Wall stress
- ↑ DO2 by ↓ PAdiast and ↑ PtdLV
- Insufficient capillary development in relation to contractile mass
|
References
- ARDEHALL A, SEGAL J, CHEITLIN MD. Coronary blood flow reserve in acute aortic regurgitation. J Am Coll Cardiol 1995; 25:1387-93
- BORER JS, HERROLD EM, HOCHREITER C, et al. Natural history of left ventricular performance at rest and during exercise after aortic valve replacement for aortic regurgitation. Circulation 1991; 84:III103-9
- BORROW K, GREEN LH, MANN T, et al. End-systolic volume as a predictor of postoperative left ventricular performance in volume overload from valvular regurgitation. Am J Med 1980; 68:655-60
- CARABELLO BA. Aortic regurgitation: a lesion with similarities to both aortic stenosis and mitral regurgitation. Circulation 1990: 82:1051-3
- DOWNES TR, NOMEIR AM, HACKSHAW BT, et al. Diastolic mitral regurgitation in acute but not chronic aortic regurgitation. Implications regarding the mechanism of mitral closure. Am Heart J 1989; 117:1106-12
- EUSEBIO J, LOUIE EK, EDWARDS DC, et al. Alterations in transmitral flow dynamics in patients with early mitral valve closure and aortic regurgitation. Am Heart J 1994; 128:941-8
- KAWANISHI DT, McKAY CT, CHANDRARATNA AN, et al. Cardiovascular response to dynamic exercise in patients with chronic symptomatic mild-to-moderate and severe aortic regurgitation. Circulation 1986; 73:62-9
- NITENBERG A, FOULT JM, ANTONY I, et al. Coronary flow and resistance reserve in patients with chronic aortic regurgitation, angina pectoris and normal coronary arteries. J Am Coll Cardiol 1988; 11:478
- OPIE LH. Heart Physiology. From cell to circulation. 4th edition. Philadelphia: Lippincott Williams & Wilkins 2004, 402-430
- TANIGUSHI K, NAKANO S, MATSUDA H, et al. Timing of operation for aortic regurgitation: Relation to postoperative contractile state. Ann Thorac Surg 1990; 50:779-85
