VSDs are present in 25% of paediatric congenital malformations. They are the most common heart defects in the first year of life. Their prevalence subsequently falls to 10%, since over 40% of VSDs close spontaneously during childhood [8]. The interventricular septum is muscular, with the exception of its upper part situated below the aortic valve, which is membranous. Communication between the two ventricles may occur at four different locations (Figure 14.43) [3].
- Muscular VSD: this is the most common form in young children; it is often multiple (Swiss cheese septum) and situated in the apical two-thirds of the septum (Figure 14.44A). Muscular VSD is not associated with valve dysfunction and may close spontaneously due to muscular hypertrophy of the septum (Video).
Video: Muscular VSD through the interventricular septum; poorly visible in 2D imaging, it is well apparent with colour flow.
- Membranous or perimembranous VSD (typical of tetralogy of Fallot): it may close spontaneously by progressive occlusion through redundancy of the tricuspid valve's septal leaflet. It may be associated with valve insufficiency (Video) (Figure 14.44B).
Video: Membranous VSD located under the aortic valve, and typical of tetralogy of Fallot.
- Inlet VS: part of AV canal defects, but may also be isolated. It appears beneath and between the septal leaflets of the mitral valve and tricuspid valve. It may be spontaneously occluded by the tricuspid valve's septal leaflet (Video) (Figure 14.44C).
Video: Inlet VSD, located under the tricuspid valve, and typical of AV canal.
- Infundibular or supracristal VSD: located just beneath the pulmonary valve, it communicates with the left outflow tract beneath the aortic valve. This proximity frequently causes one or two cusps of the aortic valve to prolapse (right coronary and non-coronary cusps), which results in aortic insufficiency (AI). This type of VSD is operated on as soon as possible to prevent the onset of severe AI (Video) (Figure 14.44D).
Video: Infundibular VSD, located under the aortic valve; the communication is between the left and right outflow tracts; presence of a small aortic regurgitation.
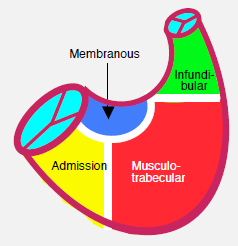
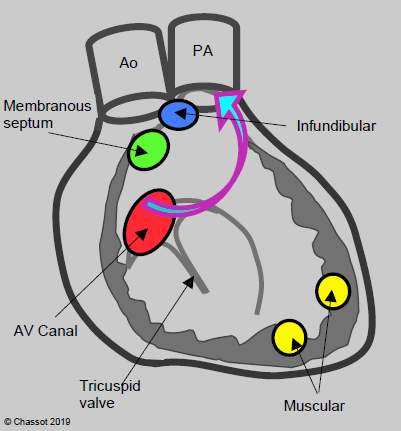
Figure 14.43: Diagram showing the various anatomical variants of ventricular septal defects (VSDs). Basal VSDs (membranous septum, AV canal defects, infundibular (or supracristal)) are located close to the RV outflow tract. Consequently, their flow bypasses the LV chamber (arrow) [3]. On the left is a diagram of the interventricular septum viewed from the RV with its various anatomical components.
In addition to their anatomical classification, VSDs are also categorised based on their haemodynamic impact (PAP: pulmonary arterial pressure. SAP: systemic arterial pressure).
- Type 1: small, restrictive VSD (Roger's disease). Qp/Qs < 1.5, PAP/SAP ratio < 0.3, PVR/SVR ratio < 0.3.
- Type 2a: wide, restrictive VSD (major shunting). Qp/Qs > 2, PAP/SAP ratio 0.3-0.7, PVR/SVR ratio 0.3.
- Type 2b: Wide, non-restrictive VSD. Qp/Qs > 2, PAP/SAP ratio 0.7-1, PVR/SVR ratio 5.
- Type 3: Eisenmenger's syndrome (wide, non-restrictive VSD). Qp/Qs < 1, PAP/SAP ratio 1, PVR/SVR ratio ≥ 0.5.
- Type 4: ASD with “protected lungs” (VSD with pulmonary stenosis). Qp/Qs > 2, PAP/SAP ratio < 0.6, PVR/SVR ratio < 0.3, RV-PA gradient > 25 mmHg.
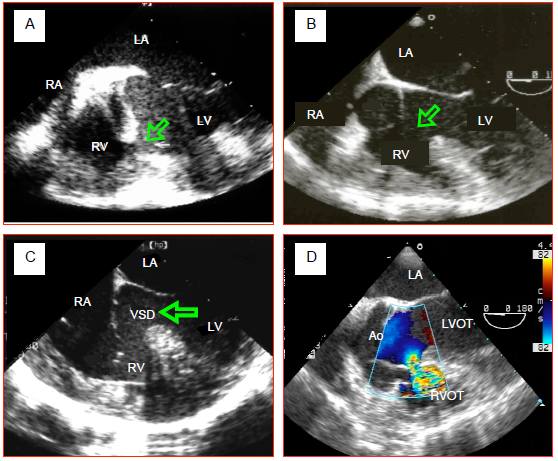
Figure 14.44: Transesophageal echocardiographic (TEE) images of VSDs. A: Muscular VSD located in the body of the interventricular septum. This image of a neat perforation is rare. Often muscular VSDs meander in the trabeculation and take a path that is difficult to follow. They may also be multiple (Swiss cheese septum). B: membranous VSD associated with tetralogy of Fallot. The aortic valve overrides the VSD. C: inlet VSD in an AV canal defect. It is located beneath the tricuspid valve in the proximal part of the interventricular septum. D: supracristal VSD located between the two outflow tracts, just beneath the aortic valve's right coronary cusp.
The VSD causes a non-cyanotic L-to-R shunt that overloads the pulmonary circulation proportionnally to its size. It represents a volume overload for the LV, which must pump the blood through the shunt into the pulmonary circulation. This volume is returned to it via the LA (see Figure 14.11B). Therefore the first issue facing children with a VSD is left-sided failure due to volume overload. If the VSD is positioned very high in the ventricular inlet and outflow tract, blood following the shunt bypasses more or less the right ventricular chamber. The LV performs the necessary work in terms of pressure and volume. The RV is involved only when pulmonary hypertension develops secondarily. This occurs comparatively early if the VSD is wide and the pulmonary system is subjected to systemic pressure. Large VSDs are the same size as the aortic annulus. They equalise pressure between the LV and RV. Since PVR is physiologically lower than SVR, systolic volume is diverted to the pulmonary vascular bed where the flow is excessive. Children become symptomatic towards their first month of life when PVR drops: tachycardia, dyspnoea, feeding difficulties, growth retardation. Unless corrected, the high pulmonary blood flow and excessive PAP leads to irreversible pulmonary vascular lesions culminating in Eisenmenger’s syndrome. In such cases, the shunt becomes bidirectional.
On echocardiographic examination, the pulmonary artery and LA are dilated as they are the shunt's inlets. The LV is dilated, oversized and hypertrophied by the volume overload as it is the driving pump for both the systemic blood flow and the VSD flow [3,5]. The RV becomes dilated and hypertrophied in proportion to the degree of pulmonary hypertension. Colour flow reveals the L-to-R crossing flow and the inlet swirl into the RV. The size of the proximal isovelocity surface area in the LV is proportional to the shunt size if this is restrictive, but it is absent if the shunt is very large, since the flow velocity and the pressure gradient through the VSD are low. In the event of major shunting, these images are observed during systole and diastole, while in small shunts, they are only observed in systole (Figure 14.45).
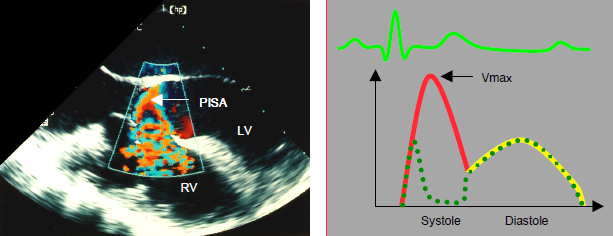
Figure 14.45: Characteristics of VSD flow. With colour Doppler, a proximal isovelocity surface area (PISA) is visible upstream of the orifice, on the left ventricular side; the swirls appear inside the RV chamber. The flow can be tracked through the VSD. On a spectral Doppler, the image shows a systolo-diastolic flow that is predominantly systolic. The flow velocity is dependent on the pressure gradient between the RV and LV. It decreases as pulmonary hypertension and right heart decompensation become established. Diastolic flow may reverse (R-to-L) in the event of right heart failure, with EDP rising higher on the right side than on the left. The dotted line shows a muscular VSD flow, with the conduit temporarily obstructed by myocardial contraction during systole.
If a VSD is present, RV systolic pressure cannot be measured accurately based on tricuspid regurgitation velocity, as the high-pressure flow from the VSD contaminates the RV pressure. However, it is possible to calculate RV systolic pressure based on the shunt flow velocity. The maximum shunt velocity (Vmax) is used to measure the pressure difference between the LV and RV during systole. When subtracted from systemic arterial pressure (APs) (assuming no aortic pathology), it gives RV systolic pressure (Ps):
RVPs = APs - 4 (Vmax VSD)2
Indications for surgical closure of the VSD vary depending on the child's age [1,2,7].
- Indications at < 6 months: congestive left ventricular failure, growth retardation. Mortality is close to 5%.
- Indications at 6-24 months: left ventricular failure, development of PAH.
- Indications > 2 years: persistence of a moderate or major shunt (Qp/Qs > 1.5:1), volume overload for the LV or pressure overload for the RV. The mortality rate is < 1%.
- Other indications: lesions associated with a complex anatomical syndrome (AV canal defect, tetralogy of Fallot), lesions in the outflow tract due to the risk of aortic insufficiency.
- Perioperative mortality is very high if PVR is > 6-8 Wood U/m2 or if the PVR/SVR ratio is > 0.3.
- At < 1 month or < 1 kg, it may be preferable to band the PA pending the reduction in PVR and the child's growth.
The VSD is closed with a patch. Most lesions may be operated on via the right atrium (transtricuspid route) to avoid a ventriculotomy that would subsequently cause refractory ventricular arrhythmias. Supracristal VSDs are accessed through the root of the PA. Only apical muscular VSDs should be accessed through the RV wall. Many muscular VSDs and some perimembranous VSDs can be occluded percutaneously with a very low morbidity and mortality rate (1-2%) [6].
The most common complications post-correction are: aortic or tricuspid insufficiency, residual VSD, obstruction of an outflow tract, and conduction blocks. Long-term sequelae of ventriculotomy are right heart dysfunction and ventricular arrhythmias that are difficult to resolve. In non-muscular VSDs, bundle branch blocks and complete AV blocks requiring the installation of a temporary or permanent pacemaker are common postoperatively (10% of cases) due to the proximity of the conduction bundles [6]. A correction performed in the first two years will preserve ventricular function and safeguard normal pulmonary circulation.
Anaesthesia
The technique is selected according to the extent of shunting and pulmonary pressure [4].
- Restrictive VSD with minor L-to-R shunting. Since PAP is normal or not very high, the aim is to create appropriate conditions for reducing shunt flow: arterial vasodilation and pulmonary vasoconstriction. Induction can be performed with halogenated agents and general anaesthesia (isoflurane) under slight hypercapnia, low FiO2 (0.3) and moderate PEEP. Extubation is performed quickly. In non-cardiac surgery, neuraxial blockade fully meets the required conditions.
- Non-restrictive VSD with major L-to-R shunting. Due to ventricular failure and limited cardiac reserve, narcotic-based anaesthesia is required (fentanyl 50-75 mcg/kg in total), with inotropic and vasodilator support (dobutamine, epinephrin-milrinone). Excessive pulmonary flow is reduced by increasing PVR: hypercapnia (hypoventilation, 2-4% CO2 added to inspired gases), low FiO2 (0.21-0.3) and PEEP (5-10 cm H2O). The adequacy of systemic flow is monitored by blood-gas analysis (respiratory acidosis) and SVO2.
- VSD with PAH – due to a bidirectional shunt through the VSD, SVR must be raised with a systemic vasoconstrictor and PVR must be lowered with a pulmonary vasodilator. Pulmonary hypertensive crisis: hyperventilation, anaesthesia deepened (fentanyl), alkalosis, epoprostenol, NO•. After correcting the VSD, the pulmonary musculature remains hyperreactive to the usual triggers (acidosis, hypoventilation, stress, pain, hypothermia, etc.).
- The increase in pulmonary flow increases the work of breathing and resistance in the airways. It reduces pulmonary compliance. There is a high risk of pulmonary infections.
Children with VSDs are highly sensitive to hypovolaemia. Although their circulating volume is increased, some of this volume is simply circulating through the lungs by the shunt. This portion is unavoidable: it is dependent on the orifice diameter and is subjected to lower pressure than the systemic pressure. A loss of circulating volume therefore occurs mainly at the expense of volume circulating in the peripheral systemic circulation because of the "steal" phenomenon through the shunt and the lungs. Arterial vasoconstriction due to hypovolaemia merely prompts a greater proportion of volume to flow into the shunt’s circuit at the expense of systemic perfusion.
Weaning from CPB is generally easy in cases of restrictive VSDs, since, once relieved of its volume overload, the LV is rather hyperdynamic. However, difficulties may arise in terms of pulmonary hypertensive crises and right heart failure if the VSD is very wide. In such cases, PVR must be lowered (hyperventilation, NO•) and inotropic support must be provided (dobutamine, milrinone). After a VSD has been corrected by a patch, a small residual leak is tolerable, provided that: it is minor (diameter < 2 mm), no flow convergence area appears on the left side, and the enrichment of O2 saturation in the PA is < 5% [9]. In the event of multiple VSDs, it is not uncommon for smaller defects to appear only after the closure of the main lesion (preferential shunt). A thorough echocardiographic examination of the tricuspid valve is recommended, since the septal leaflet may undergo tethering on closure of a perimembranous or inlet VSD. Moreover, the right transatrial repair technique requires partial detachment of the tricuspid valve, which must then be reconstructed. The degree of residual leakage must be clearly assessed. During repairs of infundibular VSDs, the right coronary cusp or non-coronary cusp of the aortic valve may be retracted, resulting in aortic insufficiency.
The most common complications are residual shunting (6-10% of cases), PAH crises, and RV or LV dysfunction. An AV block occurs in 10% of cases. Junctional ectopic tachycardia (JET) is an arrhythmia found specifically in young children and is difficult to interrupt. It is normally overcome with local cooling combined with amiodarone or procainamide [2].
Weaning from CPB is generally easy in cases of restrictive VSDs, since, once relieved of its volume overload, the LV is rather hyperdynamic. However, difficulties may arise in terms of pulmonary hypertensive crises and right heart failure if the VSD is very wide. In such cases, PVR must be lowered (hyperventilation, NO•) and inotropic support must be provided (dobutamine, milrinone). After a VSD has been corrected by a patch, a small residual leak is tolerable, provided that: it is minor (diameter < 2 mm), no flow convergence area appears on the left side, and the enrichment of O2 saturation in the PA is < 5% [9]. In the event of multiple VSDs, it is not uncommon for smaller defects to appear only after the closure of the main lesion (preferential shunt). A thorough echocardiographic examination of the tricuspid valve is recommended, since the septal leaflet may undergo tethering on closure of a perimembranous or inlet VSD. Moreover, the right transatrial repair technique requires partial detachment of the tricuspid valve, which must then be reconstructed. The degree of residual leakage must be clearly assessed. During repairs of infundibular VSDs, the right coronary cusp or non-coronary cusp of the aortic valve may be retracted, resulting in aortic insufficiency.
The most common complications are residual shunting (6-10% of cases), PAH crises, and RV or LV dysfunction. An AV block occurs in 10% of cases. Junctional ectopic tachycardia (JET) is an arrhythmia found specifically in young children and is difficult to interrupt. It is normally overcome with local cooling combined with amiodarone or procainamide [2].
| Ventricular septal defects (VSDs) |
|
Characteristics:
- Non-cyanotic L → R shunt, increased pulmonary blood flow (Qp ↑, Qp/Qs > 1.5) - Dilated receiving chambers: PA, LA, LV - Volume overload for the LV - Restrictive shunt: PAP at the upper limit of normal levels - Non-restrictive shunt: PAH, secondary pressure overload for the RV, RVH Management: reduce shunting by lowering SVR and increasing PVR Anaesthesia recommendations: - Ventilation: FiO2 0.3, hypercapnia (except if PAH present), low ventilation pressure - Anaesthesia: halogenated agent (isoflurane) - Hypovolaemia poorly tolerated due to the sequestration of volume in the pulmonary circulation - If PAH: hyperventilation, NO, alkalosis, fentanyl - Post-op: early extubation (if normal PAP), risk of AV block |
© BETTEX D, BOEGLI Y, CHASSOT PG, June 2008, last update February 2020
References
- ABMAN SH, HANSMANN G, ARCHER SL, et al. Pediatric pulmonary hypertension. Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132:2037-99
- BENT ST. Anesthesia for left-to-right shunt lesions. In : ANDROPOULOS DA, et al, eds. Anesthesia for congenital heart disease. Oxford: Blackwell-Futura, 2005, 297-327
- BETTEX D, CHASSOT PG. Transesophageal echocardiography in congenital heart disease. In: BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1186-1212
- CHASSOT PG, BETTEX DA. Anesthesia and adult congenital heart disease. J Cardiothorac Vasc Anesth 2006; 20:414-37
- CHASSOT PG, BETTEX D. Perioperative transoesophageal echocardiography in adult congenital heart disease. In: POELAERT J, SKARVAN K. Transoesophageal echocardiography in anaesthesia. London, BMJ Book, 2004
- HONJO O, Van ARSDELL GS. Cardiovascular procedures : surgical considerations. In : BISSONNETTE B, edit. Pediatric anesthesia. Basic principles, State of the art, Future. Shelton (CO): People’s Medical Publishing House (USA), 2011, 1589-608
- LUN K, LI H, LEUNG MP. Analysis of indications for surgical closure of subarterial ventricular septal defect without aortic cusp prolapse and aortic regurgitation. Am J Cardiol 2001; 87:1266-70
- PERLOFF JK: Survival patterns without cardiac surgery or interventional catheterization: a narrowing base, in: PERLOFF JK, CHILD JS, (eds): Congenital heart disease in adults. 2nd edition. Philadelphia, WB Saunders, 1998, 15-53.
- TEE SDG, SHIOTA T, WEINTRAUB R, et al. Evaluation of ventricular septal defect by transesophageal echocardiography: Intraoperative assessment. Am Heart J 1994; 127:585-92
