TGA entails complete discordance of the ventriculoarterial junction, with the aorta stemming from the anatomically right ventricle acting as the systemic ventricle, and the pulmonary artery stemming from the morphologically left ventricle. In dextro-TGA (D-TGA), the two vessels run parallel – the aorta is anterior and the pulmonary artery is posterior (Figure 15.53). Levo-TGA (or L-TGA) is combined with ventricular discordance. The aorta and PA are parallel, but side-by-side in the frontal plane (see Congenitally corrected TGA) [11].
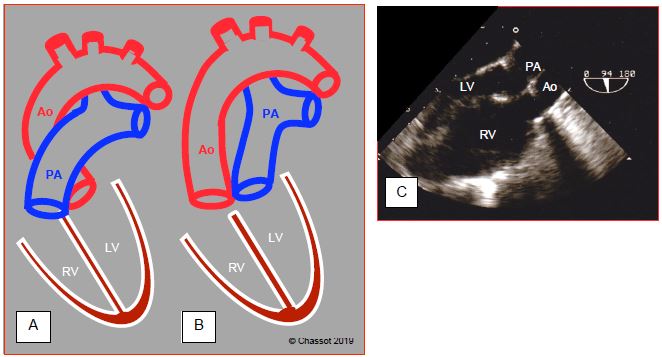
Figure 15.53: Diagram showing transposition of the great arteries (TGA). A: normal position of the aorta and pulmonary artery. B: position of vessels in D-TGA. C: long-axis view of the aorta and PA, which appear parallel instead of crossing at 45°. The anatomically right ventricle is hypertrophied since it acts as a systemic ventricle (sub-aortic). The aorta is anterior to the PA.
The systemic and pulmonary circulations operate in parallel rather than in sequence, which is incompatible with survival. A mixture of venous and arterialised blood through an ASD, VSD (30-40% of cases) or patent ductus arteriosus ensures survival. Children with TGA do not survive unless surgery is performed at an early stage.

Figure 15.53: Diagram showing transposition of the great arteries (TGA). A: normal position of the aorta and pulmonary artery. B: position of vessels in D-TGA. C: long-axis view of the aorta and PA, which appear parallel instead of crossing at 45°. The anatomically right ventricle is hypertrophied since it acts as a systemic ventricle (sub-aortic). The aorta is anterior to the PA.
The systemic and pulmonary circulations operate in parallel rather than in sequence, which is incompatible with survival. A mixture of venous and arterialised blood through an ASD, VSD (30-40% of cases) or patent ductus arteriosus ensures survival. Children with TGA do not survive unless surgery is performed at an early stage.
- Rashkind procedure: the atrial septum is perforated by catheterisation to create or enlarge the ASD. This procedure is performed within hours of birth.
- Mustard or Senning procedure: a complex patch positioned between the two atria (interatrial baffle) redirects systemic venous blood to the sub-pulmonary ventricle and arterialised blood to the sub-aortic ventricle (Figure 15.54). Unfortunately, the anatomically right ventricle is still the systemic ventricle, preventing normal survival. It becomes dysfunctional around the age of 20-25 years [7].
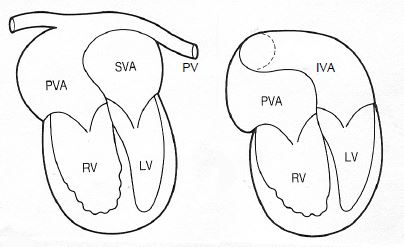
Figure 15.54: Mustard procedure for TGA: a complex patch positioned between the two atria (interatrial baffle) redirects systemic venous blood to the sub-pulmonary ventricle (LV) and arterialised blood to the sub-aortic ventricle (RV). The systemic ventricle is the right ventricle. A: the pulmonary veins (PV) drain into a posterior venous atrium (PVA) connected to the sub-aortic RV. B: the inferior vena cava (IVC) drains into the lower part of an anterior venous atrium (inferior venous atrium IVA) connected to the sub-pulmonary LV. In the same way, the superior vena cava drains into the upper part of the anterior venous atrium (superior venous atrium SVA) [13].
- Jatene procedure (arterial switch): before significant remodelling of the ventricles occurs (< 2-3 weeks of life), the great arteries are switched by reconnecting the PA to the RV and the aorta to the LV. The coronary arteries are reimplanted in the aorta (Figure 15.55). Once the risk of perioperative mortality (3%) has passed, life expectancy is virtually normal (97% at 20 years) [9,10,16].
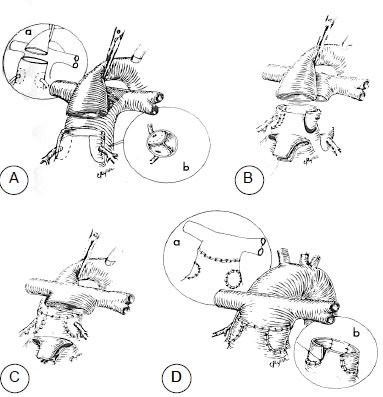
Figure 15.55: Jatene procedure (arterial switch): switching of the great arteries by reconnecting the PA to the RV and the aorta to the LV. A: transection of the aorta and excision of the coronary arteries. B: the PA is excised and the coronary arteries are reimplanted in the proximal part of the PA connected to the LV. C: The PA is positioned anteriorly to the aorta (Lecompte manoeuvre). The proximal PA is anastomosed to the distal aorta. D: the sites from which the coronary arteries have been removed are patched and the proximal aorta, which is connected to the RV, is anastomosed to the distal PA. This reconstruction entails a risk of myocardial ischaemia.
- Rastelli procedure: the anatomically left ventricle is connected to the aorta by a patch over the VSD and the anatomically right ventricle is connected to the pulmonary artery by a valved conduit (Figure 15.56). It is only used in complex cases with VSD.
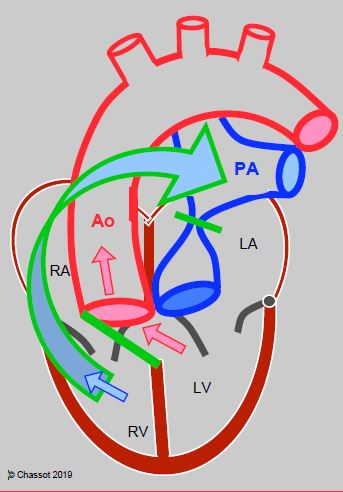
Figure 15.56: Rastelli procedure: if TGA is associated with a VSD and an obstruction of the LVOT, the VSD may be closed with a patch (green line) positioned so that blood from the LV is directed towards the aorta. The proximal PA is ligated and a valved conduit (green/blue arrow) is built between the RV and PA. The venous blood (blue arrow) and arterial blood (red arrows) is separated and directed respectively to the PA conduit and the aorta.
The oldest adults have generally undergone a Mustard or Senning-type atrial switch, while those born in the nineteen eighties have already received an arterial switch.
Anaesthesia
Adult patients have therefore already undergone surgery, but nevertheless do not exhibit normal haemodynamics.
- Following an atrial switch (Mustard or Senning procedures), right ventricular dysfunction is the norm. The RV is dilated and tricuspid insufficiency appears [5]. The LV is small and the interventricular septum bulging to the left further reduces its diastolic volume. An obstruction of the systemic and/or pulmonary venous return by the interatrial patch is present in > 25% of cases. This can cause SVC syndrome (most common), protein-losing enteropathy, or pulmonary stasis [15]. Atrial tachyarrhythmias, sinus bradycardia and AV blocks are common (50-60% of cases) and refractory to drug treatment [2,8]. Dynamic obstruction of the LVOT is possible since the LV is connected to a low-pressure circuit. During anaesthesia, the anatomically right ventricle requires inotropic support and optimal coronary perfusion as it ensures systemic perfusion.
- Post-arterial switch, physiology is virtually normal. The clinical picture is dominated by two complications: aortic root dilation (> 50 mm) with neoaortic valve insufficiency (30% of cases) and ischaemia following coronary artery reimplantations (5-10% of cases) [4,6,14]. Dynamic obstructions of the RVOT or LVOT and LV dysfunction may also be encountered [3]. These patients are sensitive to hypovolaemia and beta-stimulation, but otherwise exhibit normal haemodynamic reactivity [4,16].
- Following a Rastelli procedure, a residual VSD may cause volume overload for the LV. Secondary stenosis of the conduit must be corrected if the maximum pressure gradient is > 50 mmHg or the RV/LV pressure ratio > 0.7 [1,12,15].
| Transposition of the great arteries (TGA) |
|
Patients undergo surgery during infancy (they would not survive without treatment or palliation).
Switch of the venous returns (Mustard and Senning procedures). - The RV continues to be the systemic ventricle, hence right heart failure around 20-30 years - Dynamic obstruction of the LVOT - Venous stasis - Arrhythmias Arterial uncrossing (Jatene procedure, arterial switch). - AI (neoaortic valve) - Myocardial ischaemia (coronary artery reimplantation) - Dynamic obstruction of the LVOT and/or RVOT Implications for anaesthesia - Post-atrial switch: right-sided failure (systemic ventricle), atrial arrhythmias, venous stasis - Post-arterial switch: normal physiology; risk: AI, coronary ischaemia |
© BETTEX D, CHASSOT PG, January 2008, last update May 2018
References
- BAUMGARTNER H, BONHOEFFER P, DE GROOT NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010; 31:2915-57
- CHRISTENSEN RE, REYNOLDS PI, BUKOWSKI BK et al. Anaesthetic management and outcomes in patients with surgically corrected D-transposition of the great arteries undergoing non-cardiac surgery. Br J Anaesth 2010; 104:12-5
- COLAN SD, BOUTIN C, CASTANEDA AR, WERNOVSKY G. Status of the left ventricle after switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 1995; 109:311-21
- COLAN SD, TROWITZSCH E, WERNOVSKY G, et al. Myocardial performance after arterial switch operation for transposition on the great arteries with intact ventricular septum. Circulation 1988; 78:132-41
- DIMAS AP, MOODIE DS, STERBA R, GILL CC. Long-term function of the morphologic right ventricle in adult patients with corrected transposition of the great arteries. Am Heart J 1989; 118:526-30
- FORMIGARI R, TOSCANO A, Giardini A, et al. Prevalence and predictors of neoaortic regurgitation after arterial switch operation for transposition of the great arteries. J Thorac cardiovasc Surg 2003; 126:1753-9
- GRAHAM TP. Ventricular performance in congenital heart disease. Circulation 1991; 84:2259-74
- HELBING WA, HANSEN B, OTTENKAMP J, et al. Long-term results of atrial correction for transposition of great arteries: comparison of Mustard and Senning operations. J Thorac Cardiovasc Surg 1994; 108:363-72
- HOVEL-GURICH HH, SEGHAYE MC, MA Q, et al. Long-term results of cardiac and general health status in children after neonatal arterial switch operation. Ann Thorac Surg 2003; 75:935-43
- MASCIO CE, PASQUALI SK, JACOBS JP, et al. Outcomes in adult congenital heart surgery: analysis of the Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2011; 142:1090-7
- MILLER-HANCE WC, SILVERMAN NH. Transesophageal echocardiography (TEE) in congenital heart disease with focus on the adult. Cardiol Clinics 2000; 18:861-92
- SILVERSIDES CK, SALEHIAN O, OECHSLIN E, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Complex congenital cardiac lesions. Can J Cardiol 2010; 26:e98-e117
- STÜMPER O, SUTHERLAND GR. Transesophageal echocardiography in congenital heart disease. London: Edward Arnold, 1994, 37-38
- TANEL RE, WERNOWSKY G, LANDZBERG MJ, et al. Coronary artery abnormalities detected at cardiac catheterization following the arterial switch operation for transposition of the great arteries. Am J Cardiol 1995; 76:153-
- WARNES CA, WILLIAMS RG, BASHORE TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008; 118:2395-451
- WILLIAMS WG, McCRINDLE BW, ASHBURN DA, et al. Outcomes of 829 neonates with complete transposition of the great arteries 12-17 years after repair. Eur J Cardiothorac Surg 2003; 24:1-9
