When weaning off time comes, heart rhythm is very often unstable for several reasons (see Chapter 20 Types of arrhythmias) [1].
- Residual hyperkalaemia from cardioplegia causes variable conduction blocks;
- The inhomogeneity of the return to normal conduction after cardioplegia and hypothermia is key for reentry arrhythmias (AF, VT);
- Acute ischaemia (coronary embolism of air or atheromatous fragments) leads to various forms of ventricular arrhythmias;
- After adequate coronary revascularisation, reperfusion arrhythmias may be particularly resistant to treatment;
- Surgical damage to the conduction tissue leads to momentary or permanent block; the incidence is up to 10% after aortic valve replacement [80];
- Beta stimulation with inotropic agents is arrhythmogenic.
Atrial fibrillation (AF) is common after cardiac surgery (10-40% of cases). It suppresses the propulsive activity of the atrium and lowers cardiac output by decreasing the end-diastolic preload of the ventricle. Sinus heart rhythm preserves better electrical stability, reduces the risk of systemic embolism and may avoid anticoagulation, even if associated with dilated and poorly contracting atria marginally effective in haemodynamic [4]. Treatment of AF is indicated for patients who were sinusal prior to the procedure and for those who are haemodynamically intolerant [6].
- Cardioversion (2-10 J by internal pads, 50-150 J if transthoracic) ;
- Magnesium (MgCl2 10-20 mmoles or 2-4 g iv);
- Amiodarone (Cordarone®5-5 mg/kg iv over 20 min, then 15 mg/kg over 24 hours);
- Esmolol (Brevibloc®5 mg/kg iv over 1 min; infusion 0.05-0.2 mg/kg/min);
- Vernakalant (Brinavess® ) (3 mg/kg iv in 10 min), the first class I and III atrial selective substance to be effective after cardiac surgery.
It is usual for the heart to fibrillate after aortic declamping. This fibrillation may resolve spontaneously, but it is usual to defibrillate the patient with a 5-10 J shock using internal pads. If the first shock is ineffective, Mg2+ (2-4 g) and lidocaine (1.5 mg/kg) are added via the central line or ECC line. The power is increased to 50 J (maximum) during iterative shocks. Amiodarone is the first pharmacological choice in case of instability: loading dose 150-300 mg slow iv, followed by an additional 150 mg infusion [5]. It is important to normalise all metabolic elements: electrolytes, pH, blood glucose, ventilation, etc. Occasionally, myocardial reperfusion injury can result in recurrent tachycardia and ventricular fibrillation. It is essential to keep defibrillating the patient, even dozens of times if necessary, because these reperfusion injuries are completely reversible within a few hours. Refractory fibrillation can also be interrupted by cardioplegia, but it requires the patient to return to ECC.
Arrhythmias and blocks often involve electro-systolic training. As arrhythmias can be triggered suddenly at any time in the postoperative period, it is usual to insert epicardial electrodes connected to a pacemaker after any cardiac operation. The electrodes are attached to the epicardium, avoiding fatty deposits. Two setups are possible.
- Unipolar system: the anode (negative pole) is attached to the epicardium and the cathode in the subcutaneous tissue;
- Bipolar system: anode and cathode are placed very close to each other on the epicardium; this system requires less stimulation energy; it is less sensitive to electrical interference.
The epicardial electrode is implanted on the right ventricle (RV) because the haemodynamic outcome is superior to apical placement: improvement of EF, normalisation of the QRS axis, preservation of LV torsional motion [3]. The only exception is obstructive hypertrophic cardiomyopathy (OHC) where pacing the plenum may result in dynamic obstruction in systole. In cases of ventricular dilation and conduction delay, placement of two pacing electrodes, one on the RVOT and one posterobasal on the LV, allows resynchronisation of both ventricles, but temporary external pacemakers do not have the regulatory capabilities of implanted devices (see Chapter 20 Resynchronizing).
Pacemaker Setting
Pacemaker settings differ from case to case, but most often vary around the same basic values (see Chapter 20, Settings) [2].
- Frequency: 80 beats/min; in case of spontaneous rhythm, 10 beats/min above the intrinsic frequency;
- AV interval: 150 ms; 100-225 ms depending on the best preload increase by atrial contraction;
- Activation energy (output) at twice the observed stimulation threshold; max: 15 mA ;
- Sensing at half the threshold, or 2 mV;
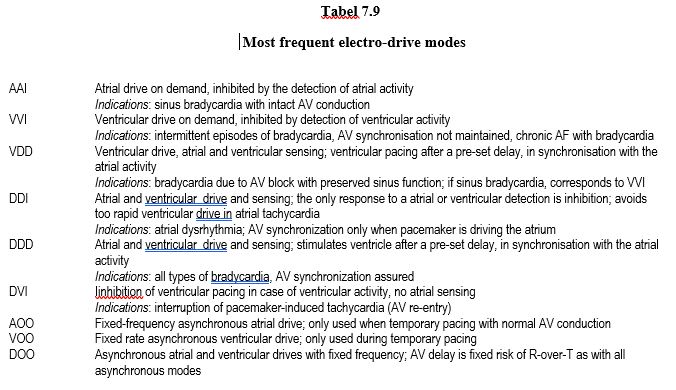
- Typical pacing modes of temporary external pacemakers (Table 7.9) :
- DOO (fixed frequency asynchronous atrial and ventricular drives) ;
- VOO (fixed frequency asynchronous ventricular drive) ;
- DVI (inhibition of ventricular pacing in case of ventricular activity, no atrial sensing) ;
- VVI (ventricular drive on demand, inhibited by the detection of ventricular activity) ;
- DDD (atrial and ventricular pacing and sensing; stimulates the ventricle after a pre-set delay, in synchronisation with atrial activity).
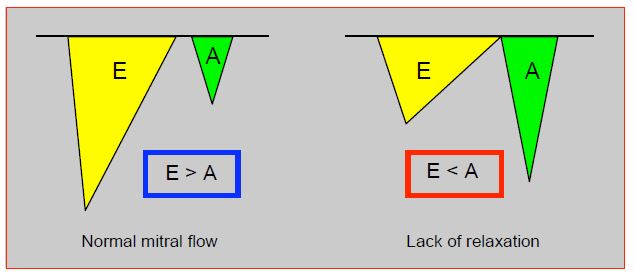
Sinus rhythm is always better than ventricular rhythm, as atrial contraction increases stroke volume by 20-45%. By measuring the size of the A component of mitral flow (area under the curve), trans-oesophageal echocardiography is very useful to assess the effectiveness of atrial contraction and set the best AV delay (Figure 7.50).
Figure 7.50: Mitral flow as seen on TEE. The protodiastolic E flow is related to active LV relaxation, the A flow is due to atrial contraction. The area under the curve of each E and A component is proportional to the amount of blood flowing through the mitral. Normally, A-flow contributes only 20% of the ventricular filling (E > A). In diastolic dysfunction due to relaxation failure, the E component decreases and the atrial component becomes more important, contributing 30-45% of LV end-diastolic filling; A flow is more important than E flow (E < A). Patients with this type of diastolic dysfunction benefit greatly from sinus rhythm. TEE is very useful for judging the haemodynamic adequacy of the heart rhythm and for guiding pacemaker setting (pacing mode, AV delay).
If atrial contraction is haemodynamically ineffective (atrial electro-mechanical dissociation), there is no need to insist on maintaining sinus rhythm, apart from electrical stability and anticoagulation necessitySS.
In order to test the pacing thresholds (output), the rate is set to ≥ 10 beats/min above the patient's spontaneous frequency and sensitivity to its maximum value. The pacing power (mA) is gradually increased until the relevant chamber captures the pacing and displays a QRS complex on the ECG. When an atrial lead is tested, the tracing shows a fine sinus-like QRS pattern, whereas the image is that of a wide QRS (ESV type) when a ventricular lead is tested. In both cases, the heart rate undergoes an abrupt rate jump of ≥ 10 beats/min. If the thresholds are too high, the electrodes are reversed. The pacing power is then set at twice the observed threshold, but not > 15 mA.
Sensing is the setting that allows the pacemaker to pick up the lowest possible electrical signal from the heart. A low threshold corresponds to a high sensitivity. In order to test it, frequency is set 10 beats/min below the patient's spontaneous frequency, the emission setting (mA) is set to minimum and sensitivity setting is set to its highest value (10-12 mV); the pacemaker, which is in VVI, AAI or DDD mode, does not pace. As the sensitivity is progressively decreased, the corresponding light starts to flash for each R-wave. The sensitivity is then set to half the observed threshold. If the patient has no rhythm of his own, the sensitivity is preset to 2. Another way to proceed is to start with the lowest sensing value and gradually increase it, thus making the pacemaker less sensitive, until the light stops flashing and the pacemaker paces asynchronously [2].
| Pacemaker setting |
| Epicardial electrodes and temporary external pacemaker after any cardiac surgery. Monopolar electrode (active anode on the epicardium, cathode in the subcutaneous) or bipolar (anode and cathode 1-2 cm apart on the electrode)
Threshold: minimum power value (output) at which the pacemaker drives the relevant cavity (< 15 mA)
Sensing: minimum amplitude of signals that the pacemaker recognises as cardiac electrical activity (default setting: 2 mV)
Frequency: 70-80 beats/min
AV synchronization: successive stimulations of the atrium and ventricle; average delay: 150 msec
|
© CHASSOT PG, GRONCHI F, April 2008, last update, December 2019
References
- CHIOLERO R, BORGEAT A, FISCHER A. Postoperative arrhythmias and risk factors after open heart surgery. J Thorac Cardiovasc Surg 1991; 39:81-4
- CHUA J, SCHWARZENBERGER J, MAHAJAN A. Optimization of pacing after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2012; 26:291-301
- DE COCK CC, GIUDICI MC, TWISK JW, et al. Comparison of the haemodynamic effects of right ventricular outflow tract pacing with right ventricular apex pacing. A quantitative review. Europace 2003; 5:275-8
- MAESEN NIJS J, MAESSEN J, et al. Postoperative atrial fibrillation: a maze of mechanisms. Europace 2012; 14:159-74
- NEUMAR RW, OTTO CW, LINK MS, et al. Part 8: Adult Advanced Cardiovascular Life Support: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010; 122:S729-S767
- PHILIP I, BERROËTA C, LEBLANC I. Perioperative challenges of atrial fibrillation. Curr Opin Anesthesiol 2014; 27:344-52