In ischaemic mitral insufficiency, the leaflets are normal and regurgitation reflects an underlying problem in the ventricle. This MI is caused by several mechanisms (see Figure 11.65) [25].
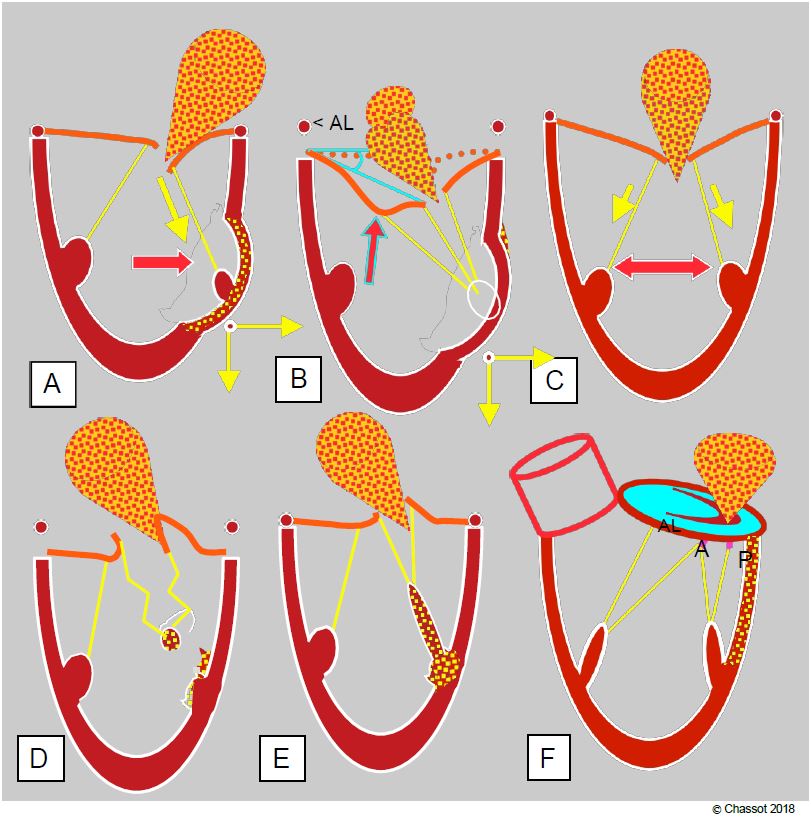
- Asymmetric restriction of the systolic stroke of the leaflets (restrictive MI type IIIb) due to segmental akinesia or dyskinesia of the ventricular wall resulting in lateral and/or apical displacement of a papillary muscle. Excessive traction on the secondary chords during lateral displacement may deform the anterior leaflet, resulting in a "gull wing" appearance on echocardiography (Figure 11.65B). Posterior leaflet narrowing is more likely to be associated with inferior infarction.
Video: Secondary mitral insufficiency on LV dilatation due to ischaemic cardiomyopathy with extensive anterolateral akinesia resulting in a slightly asymmetric MI.
- Symmetric restriction in dilatative cardiomyopathy of ischaemic origin (restrictive MI type IIIb) with significant LV dilatation.
Video: Secondary mitral insufficiency on LV dilatation due to ischaemic cardiomyopathy with extensive anterolateral akinesia resulting in a slightly asymmetric MI.
- Tilting of one or more leaflets due to papillary rupture or ischaemia; partial or complete rupture of a leaflet leads to tilting of the corresponding commissure into the LA during systole (type II MI) and massive regurgitation (see Figure 26.15). Non-contraction of a papillary muscle in systole results in excessive length of the chords and the point of coaptation of the leaflets is tilted into the LA (type II MI).
Video: Total rupture of the anterior papillary muscle, a fragment of which oscillates at the end of the chords of the anterior leaflet; the mitral valve remains open in systole.
Video: Prolapse of the anterior commissure on ischaemia-rupture of the anterior papillary muscle (70° bicommissural view); the LV and LA are dilated.
Video: Prolapse of the anterior commissure on ischaemia-rupture of the anterior papillary muscle (4-cavity view 0°); eccentric MI is severe (type II MI).
- Dilatation and flattening of the mitral annulus due to akinesia of the inferior and posterior basal wall (type I MI); annular dilatation is asymmetric; it mainly affects the posterior part of the annulus because the anterior part is attached to the fibrous trine.
Video: moderate central mitral insufficiency (type I); the jet is symmetrical and reaches the posterior wall of the LA.
Ischaemic MI is not uncommon, occurring in 10-40% of patients with coronary artery disease undergoing surgery [3]. In 76% of cases, it is of the restrictive type (IIIb). As it is the signature of a severe LV lesion, it significantly worsens the prognosis: the mortality of coronary revascularisation combined with mitral repair is approximately double that of simple revascularisation (mean < 2%) [10,12,27,29]. It is true that residual MI is greater after ECC alone, but the additional correction of moderate to severe MI does not benefit long-term outcome or survival [27,29]. Indeed, mitral repair is a valvular response to a ventricular problem !
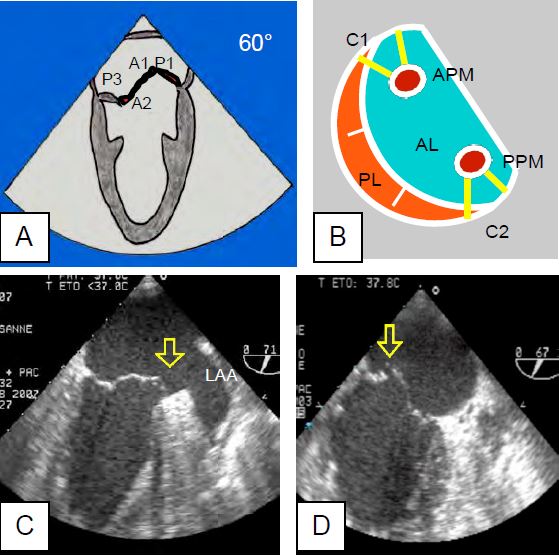
Figure 26.15 : Ischemia or partial rupture of the anterior leaflet. A: In the bicommissural view, the entire anterior commissure is tilted (prolapse of A1 and P1). B: This can be explained by the fact that the pillars are anatomically centred on the commissures and send chords to the 2 leaflets. APM: anterior papillary muscle. PPM: posterior papillary muscle. AL: Anterior leaflet. PL: posterior leaflet. C: Prolapse of the anterior commissure due to ischaemia of the anterior wall of the LV. D: Posterior commissure prolapse due to partial rupture of the posterior pillar.
Indications for surgery
The detection of a moderate-to-severe or severe MI during coronary artery bypass grafting (CABG) by the anaesthetist echocardiographer poses a difficult problem in terms of the indication for surgery, since valve replacement increases the mortality rate of CABG by a factor of 3-5, which can be as high as 15% in poor cases. The analysis of TEE images is crucial for the surgical decision [14]. Current recommendations for concomitant mitral valve surgery during coronary revascularisation are rather restrictive [2,14,19,20,22,24].
- Moderate or moderate-to-severe secondary ischaemic MI: uncertain benefit, generally no indication for surgery.
- Severe and symptomatic secondary ischaemic MI (stage D): indication depends on the situation.
- Class IIa indication when the procedure is straightforward, the EF is >30%, the LV is not severely dilated, the operative risk is low and the patient's long-term survival is highly likely.
- Class IIa indication if MI worsens on stress test; conversely, a reduction in MI on dobutamine indicates LV contractile reserve that favours revascularisation alone without concomitant mitral intervention.
- Severe MI due to papillary muscle rupture: indication for emergency repair (usually prosthetic valve replacement).
Three important factors must be considered when determining the degree of ischaemic MI intraoperatively.
- Ischaemic MI is a highly dynamic phenomenon that changes as a function of LV afterload, size and contractility.
- Anaesthesia and positive pressure ventilation alter the loading conditions and reduce the size of the MI; normal haemodynamics (MAP ≥ 80 mmHg) must be restored, if necessary with vasopressors, before a quantitative assessment can be made.
- The quantification scale for ischaemic MI is more restrictive than that for organic MI (Table 11.10); ischaemic MI is considered severe if the diameter of the vena contracta is ≥ 40 mm (instead of 70 mm for organic MI), the area of the regurgitant orifice is ≥ 20 mm2 (instead of 40 mm2 ), and the regurgitant volume is ≥ 30 mL (instead of 60 mL) [31].
The situation is different if the MI associated with coronary artery bypass grafting (CABG) is structural (primary MI) and not secondary to ischaemia. In severe MI due to leaflet pathology (degeneration, ARF, etc.), mitral valve repair or replacement is indicated according to the usual criteria for mitral valve disease. In these conditions, the combination of CABG + MVP or MVR has an operative mortality close to the sum of the two elective procedures.
Surgery
Coronary revascularisation alone can reduce MI if it is associated with active ischaemia that is reversed by CABG. If the viability of the myocardium adjacent to the columns (assessed by stress echocardiography, PET scan or MRI) and the synchronisation between the papillary muscles (assessed by tissue Doppler) are satisfactory, CABG alone, without associated plasty, is likely to reduce MI [26]. However, in 60% of cases, CABG does not correct mitral insufficiency. If the myocardial segment corresponding to the pillars is not viable and the contraction of the papillary muscles is desynchronised, revascularisation alone is not sufficient and mitral plasty is required if the MI is severe. To be meaningful, valvuloplasty should not result in an all-cause operative mortality of >4% [4].
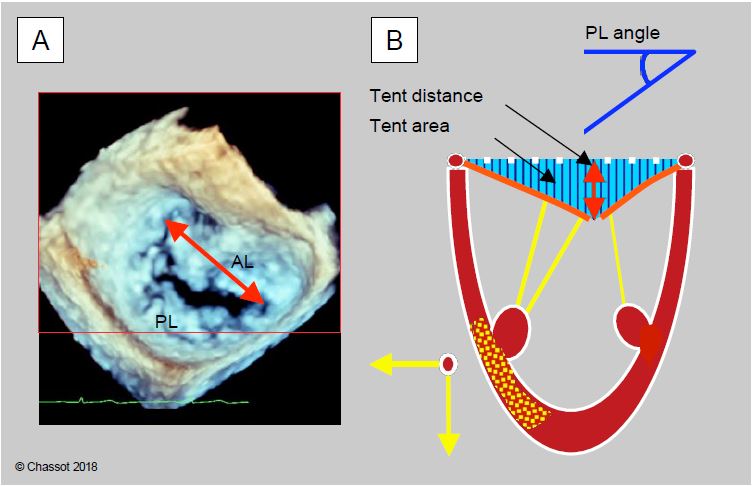
Ischaemic MI is corrected by annuloplasty (MVP with standard Carpentier-Edwards™ rigid ring or ETlogics™ and GeoForm™ rings specific for ischaemic MI), supplemented by several options depending on the situation: chord reinsertion, pillar plasty, resection of 2nd order chords, edge-to-edge confrontation. Annuloplasty is only effective if the LV dilatation is modest, as it only resolves annular dilatation but not excessive traction exerted by the chords or ventricular remodelling, which requires reconstruction of the subvalvular apparatus [19]. Certain echocardiographic data predict the persistence of mitral regurgitation or failure of annuloplasty (Figures 11.83 and 11.84) [9,11,17,20,23,28].
- Massive MI or multiple jets;
- Dilatation of the mitral annulus (diameter > 3.7 cm);
- Distance between the coaptation point and the plane of the annulus (tenting distance) > 1.1 cm;
- Tenting area between the plane of the annulus and the mitral leaflets > 2.5 cm2 (often asymmetric);
- Tenting volume between the plane of the annulus and the mitral leaflets > 3.4 cm3;
- Angle of the posterior leaflet to the plane of the mitral annulus > 45°;
- Angle between the distal end of the anterior leaflet and the plane of the mitral annulus > 25°;
- LV sphericity index (short axis diameter/long axis diameter) > 0.7;
- Papillary muscle spread in telesystole > 2.7 cm;
- LV telesystolic diameter > 5.0 cm, telediastolic diameter > 6.5 cm;
Figure 11.83: TEE measurements for feasibility of mitral valve repair. A: In 3D view, measurement of intercommissural distance. B: Echocardiographic features that determine the severity of ischaemic MI and the likelihood of conversion. The ischaemic zone displaces the column outwards and towards the apex, keeping the leaflets in the ventricular cavity during systole. The degree of remodelling induced depends on the ischaemia and any associated ventricular dysfunction; the greater the remodelling, the worse the outcome. Measures of remodelling: mitral annulus dilatation > 3.7 cm, tenting distance between the coaptation point and the plane of the mitral annulus > 1.1 cm, tenting area > 2.5 cm2 , angle of the posterior leaflet with the plane of the mitral annulus > 45°.
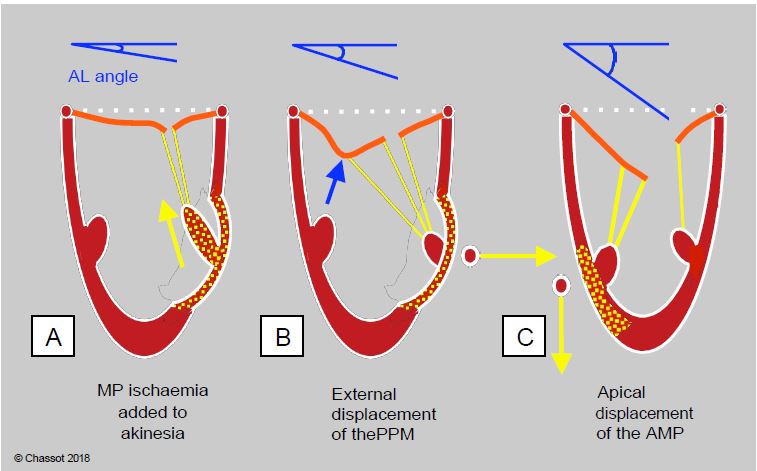
Figure 11.84: Angle of closure of the anterior leaflet (AL) of the mitral valve measured between the tip of the AL and the plane of the mitral annulus in systole. A: Ischaemia of the annulus in addition to that of the wall allows the chords to move more and the leaflets to approach their point of coaptation; the systolic angle closes and the MI decreases. B: Posterior parietal akinesia and external displacement of the posterior column; traction is predominant on the 2nd order chords because they are outwardly displaced; the anterior leaflet is angled at its centre and deformed into a gull's wing (blue arrow). Surgical resection of the chords of the 2nd order restores the situation. C: Anterior parietal akinesia and apical displacement of the anterior column; the traction is predominant in the longitudinal direction and pulls evenly on the chords; the angle of systolic closure is maximal. The larger the angle, the worse the prognosis.
These data relate to chronic ischaemic MI, which suffers from major ventricular remodelling. In acute MI, which occurs during an infarction, the dimensions of the annulus, tent and LV are much smaller, although already larger than normal [23]. On the other hand, the size of the mitral leaflets is normal and tends to increase with chronicity. This phenomenon is a compensatory adaptation in chronic MI; it is induced by excessive tension and aims to limit leakage by increasing the size of the leaflets [6,8].
The persistence of moderate-to-severe MI after the procedure, whether due to non-correction or failure of the prosthesis (15-19% of prosthesis cases), seriously compromises the functional prognosis because it worsens ventricular remodelling in proportion to its extent; up to 33% of prosthesis cases are failures at 1-2 years [11,15].
- Preload: this should remain normal-low; hypovolemia is poorly tolerated because of MI, but hypervolemia increases Vts (increase in MI) and Vtd (increase in diastolic wall tension and decrease in coronary perfusion pressure).
- Afterload: this determines the size of the MI; the aim is to achieve low SAR to reduce LV Vt, while maintaining MAP > 75 mmHg to ensure adequate coronary perfusion pressure (CPP). If a pulmonary catheter is available, this can be calculated using the formula CPP = MAP - PCWP (mmHg). Adequacy of coronary perfusion can be assessed by the following factors
- CPP ≥ 60 mmHg;
- No ECG changes;
- No new changes in segmental kinetics on TEE;
- Stable IM.
- Contractility: this must be maintained in a balance between the fall in mVO2, which favours ischaemia, and the inotropism required to reduce LV size in telesystole. Intra-aortic balloon pump is the best solution in case of ventricular failure as it reduces LV afterload, reduces MI and increases coronary perfusion.
- Rate: Slight slowing (55-60 beats/min) reduces mVO2 and prolongs diastole without increasing MI, which occurs in systole; the limit is an increase in LV size due to excess end-diastolic volume (TEE control).
- Positive pressure ventilation: this is beneficial for MI, but its haemodynamic effects vary from case to case depending on systolic and diastolic function.
- Induction: etomidate or midazolam.
- To maintain anaesthesia, take advantage of the antiischemic preconditioning offered by halogen:
- Isoflurane is most appropriate (preconditioning and lower RAS);
- Adequate sevoflurane (stable preconditioning and RAS);
- Propofol perfusion: loss of preconditioning benefit.
- Keep SARs low but avoid arterial hypotension (MAP > 75 mmHg); balance MAP with norepinephrine, nitroglycerine, isoflurane.
- Maintain normal to low blood volume.
- Special monitoring:
- TEE (changes in MI, LV size, segmental kinetics);
- Pulmonary artery catheter (PCWP, CPP, anterograde stroke volume, SvO2);
- ScO2 (adequacy of peripheral perfusion)
- Coronary sinus lactate levels (if cannulated).
| Ischaemic MI |
|
There are several causes of ischaemic MI.
- Akinesia or dyskinesia of segments adjacent to a papillary muscle (restrictive type IIIb) asymmetric)
- Ischaemic dilatative cardiomyopathy (symmetric restrictive type IIIb)
- Ischaemia or partial/total rupture of an appendage (type II)
- Basal kinesia (functional type I)
Ischaemic MI is highly dependent on haemodynamic conditions. The severity criteria for ischaemic MI are more restrictive than for organic MI.
- Vena contracta diameter ≥ 40 mm (instead of 70 mm)
- Regurgitant orifice area ≥ 20 mm2 (instead of 40 mm2)
- Regurgitant volume ≥ 30 mL (instead of 60 mL)
Indications for simultaneous mitral repair and coronary revascularisation (CABG + MVP/MVR mortality: 5-12%):
- Severe symptomatic MI (stage D) when repair is straightforward and operative risk is low
- Severe MI due to partial or total abutment rupture
- Severe organic MI (non-ischaemic)
- Moderate MI: no indication for surgery
Although it improves functional status, mitral valve repair as part of coronary revascularisation in bypass surgery does not change the prognosis for survival. In addition, the risk of recurrence is high.
Hemodynamics sought in ischaemic MI
Compromise between the constraints of coronary ischaemia and those of a large MI
Normal-low blood volume
SAR low but MAP > 75 mmHg maintained
Contractility reduced unless LV Vts increases (TEE monitoring)
Rate 60 beats/min
|
References
- ACKER MA, PARIDES MK, PERRAULT LP, et al. Mitral valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014; 370:23-32
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BONOW RO, BRAUNWALD E. Valvular heart disease. In: ZIPES DP, et al, eds. Braunwald's heart disease. A textbook of cardiovascular medicine. 7th edition. Philadelphia: Elsevier Saunders, 2005, 1553-632
- BORGER MA, ALAM A, NURPHY PM, et al. Chronic ischemic mitral regurgitation: repair, replace or rethink? Ann Thorac Surg 2006; 81:1153-61
- CHAN KMJ, PUNJABI PP, FLATHERE M, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation. Circulation 2012; 126:2502-10
- CHAPUT M, HANDSCHUMACHER MD, TOURNOUX F, et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008; 118:845-52
- CHERRY SV, JAIN P, RODRIGUEZ-BLANCO YF, FABBRO M. Noninvasive evaluation of native valvular regurgitation: a review of the 2017 American Society of Echocardiography Guidelines for the perioperative echocardiographer. J Cardiothorac Vasc Anesth 2018; 32:811-22
- DAL-BIANCO JP, AIKAWA E, BISCHOFF J, et al. Myocardial infarction alters adaptation of the tethered mitral valve. J Am Coll Cardiol 2016; 67:275-87
- DE BONIS M, AL-ATTAR N, ANTUNES M, et al. Surgical and interventional management of mitral valve regurgitation: a position statement from the European Society of Cardiology working groups on Cardiovascular Surgery and Valvular Heart Disease. Eur Heart J 2016; 37:133-9
- FERGUSON T, DZIUBAN F, EDWARDS F, et al. STS National Database: current changes and challenges for the new millenium. Ann Thorac Surg 2000; 69:680-91
- GILLINOV AM. Is ischemic mitral regurgitation an indication for surgical repair or replacement? Heart Fail Rev 2006; 11:231-9
- GILLINOV AM, WIERUP PN, BLACKSTONE EH, et al. Is repair preferable to replacement for ischaemic mitral regurgitation? J Thorac Cardiovasc Surg 2001; 122:1125-41
- GOLDSTEIN D, MOSKOWITZ AJ, GELIJNS AC, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 2016; 374:344-53
- GRAYBURN PA, CARABELLO B, HUNG J, et al. Defining "severe" secondary mitral regurgitation. Emphasizing an integrated approach. J Am Coll Cardiol 2014; 64:2792-801
- GRIGIONI F, ENRIQUEZ-SARANO M, ZEHR KJ, et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 2001; 103:1759-64
- KANG DH, KIM MJ, KANG SJ, et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation 2006; 114:1499-503
- KONGSAEREPONG V, SHIOTA M; GILLINOV AM, et al. Echocardiographic predictors of successful versus unsuccessful mitral valve repair in ischemic mitral regurgitation. Am J Cardiol 2006; 98:504-8.
- LAM BK, GILLINOV AM, BLACKSTONE EH, et al. Importance of moderate ischemic mitral regurgitation. Ann Thorac Surg 2005; 79:462-70
- LANCELLOTTI P, FATTOUCH K, LA CANNA G. Therapeutic decision-making for patients with fluctuating mitral regurgitation. Nat Rev Cardiol 2015; 12:212-9
- MAGNE J, SENECHAL M, DUMESNIL J, PIBAROT P. Ischemic mitral regurgitation: A complex multifactorial disease. Cardiology 2009; 112:244-59
- MICHLER RE, SMITH PK, PARIDES MK, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016; 374:1932-41
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- NISHINO S, WATANABE N, KIMURA T, et al. Acute versus chronic ischemic mitral regurgitation. An echocardiographic study of anatomy and physiology. Circ Cardiovasc Imaging 2018; 11:e007028
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. J Am Coll Cardiol 2017; 70:252-89
- OURY JH, CLEVELAND JC, DURAN CG; ANGELL WW. Ischemic mitral valve disease: classification and systemic approach to management. J Card Surg 1994; 9(suppl2):262-73
- PENICKA M, LINKOVA H, LANG O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation 2009; 120:1474-81
- RENEW JR, MARTIN AK, MURRAY AW, et al. Functional mitral regurgitation: interventions and outcomes. J Cardiothorac Vasc Anesth 2019; 33:2053-64
- SHAKIL O, JAINANDUNSING JS, ILIC R, et al. Ischemic mitral regurgitation: an intraoperative echocardiographic perspective. J Cardiothorac Vasc Anesth 2013; 27:573-85
- VIRK SA, TIAM DH, SRIRAVINDRARAJAH A, et al. Mitral valve surgery and coronary artery bypass grafting for moderate-to-severe ischemic mitral regurgitation. Meta-analysis of clinical and echocardiographic outcomes. J Thorac Cardiovasc Surg 2017; 154:127-36
- YUN KL, SINTEK CF, MILLER DC, et al. Randomized trial comparing partial versus complete chordal-sparing mitral valve replacement: effects on left ventricular volume and function. J Thorac Cardiovasc Surg 2002; 123:707-14
- ZOGHBI WA, ADAMS D, BONOW RO, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the ASE developed in collaboration with the SCMR. J Am Soc Echocardiogr 2017; 30:303-71