Coming after the 1/2 inch tubing connected to main pump, the arterial cannula’s tip is the narrowest point in the entire circuit (internal diameter: 7-8 mm). It is therefore the highest pressure gradient place. Normally, the gradient should not exceed 100 mmHg at a flow rate of 2.5 L/min/m2 ; beyond, the risk of haemolysis and protein denaturation is excessive. The arterial cannula has an attachment system to the vessel wall and a 3-way valve to remove air and, secondarily, to measure pressure. When the aortic cannula is in place and secured, arterial blood is allowed to flow backwards to remove debris and air bubbles through the 3 way valve. Monitoring blood pulsatility in the cannula confirms position in the aortic lumen; if the cannula is intraparietal (dissection), blood flow is not pulsatile. ECC machine cannula’s pressure should be identical to the arterial catheter pressure; any discrepancy should raise concerns about intraparietal dissection.
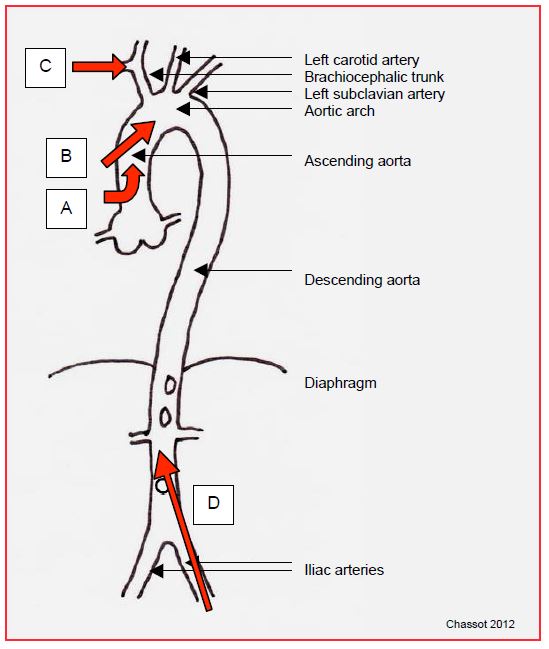
Several anatomical sites are available for arterial cannulation (Figure 7.10).
After sternotomy, the arterial cannula is usually implanted in the ascending aorta. Two types are used.
- A rigid, right-angled cannula introduced perpendicular to the anterior aortic wall, with in-line flow at the aortic root.
- Bardic™ soft cannula: introduced tangentially, flowing more distally into the arch; its position can be checked through TEE.
Cannulation of the ascending aorta presents several major risks. It is responsible by itself for much of the morbidity associated with ECC.
- Arterial embolisms: Atheromatosis of the ascending aorta occurs in 38% of patients over 50 years of age [2]; the main risk factors are advanced age, history of stroke, diabetes and peripheral vascular disease, particularly carotid disease [7]. During cannulation, atherosclerotic fragments may embolise peripherally, particularly in the brain; the risk is greatest when the plaques are ulcerated or pendulous. The transcranial Doppler brain embolism rate of 5-17% shows two very significant peaks at cannulation and aortic decanulation (see Figure 7.26) [10]. This embolisation is one factor among others responsible for postoperative strokes.
- Sandblasting effect: the blood stream exits the cannula at high velocity and blows atheromatous debris away if it is directed towards the wall and not the centre of the vessel. This effect, which is more pronounced with fine cannulas generating high velocities, is permanent throughout ECC.
Figure 7.10: Arterial cannulation sites. A: angled cannula at the root of the aorta. B: Bardic™ type cannula in the ascending aorta. C: cannula in the right subclavian artery. D: femoral cannula; in this case, the flow is retrograde in the aorta.
- Malposition of the cannula: it may be directed towards or into the brachiocephalic trunk, which thus receives all the pump flow; depending on where it is measured, the blood pressure may be high (right radial) or low (left radial and femoral). Facial colouration is asymmetric, as are carotid pulses and brain saturations (ScO2 ).
- Tears: the aortic wall is fragile in the elderly, diabetics, and atheromatous patients; the risk of a stellate tear around the cannula is greater the more the wall is strained by hypertension. It is therefore usual to lower the blood pressure during cannulation and decanulation of the aorta by aiming for a momentary MAP ≤ 50 mmHg.
- Dissection: the introduction of the arterial cannula inside the aortic wall may dissect the wall at the level of the media; this is a dramatic complication that occurs in 0.1 - 1‰ of cases [8]. It is diagnosed early during ECC by hypertension on the arterial line as the systemic pressure is collapsed and the aortic wall becomes purplish, puffy and tense. The vessels of the aortic arch may be obstructed by the false passage.
TEE, which is very good at assessing the descending aorta, can only visualise the proximal part of the ascending aorta up to its crossing with the right pulmonary artery. It is not a reliable means of demonstrating severe atheromatous lesions of the ascending aorta, because the interposition of the right stem bronchus between the oesophagus and the aorta restricts visibility to the first 4-5 cm of the ascending aorta and because most atheromas are located beyond this limit [11]. However, TEE can be used to determine the indication for epiaortic ultrasound depending on the degree of involvement of the descending aorta [6]. As surgical palpation is unreliable, only epiaortic ultrasound performed before cannulation of the aorta allows the selection of the least dangerous puncture site or modification of the cannulation (femoral or right subclavian route), in order to avoid an embolic accident if atheromatosis is too important in the ascending aorta [3,9].
In cases of dangerous atheromatosis (grade III to V atheromatosis) or extensive and friable lesions (porcelain aorta) (see Chapter 27 Aortic atheromatosis), it is recommended that manipulation of the ascending aorta be avoided and the surgical strategy adapted [1,5,7].
- Right subclavian arterial cannulation.
- Femoral artery cannulation.
- Occlusion with an intravascular balloon mounted from the femoral (Heartport™ system).
- No cardioplegia cannulation or aortic clamping: operation with beating heart or ventricular fibrillation if possible; transapical stenting if AVR.
- For coronary artery bypass grafting: multiple arterial grafts from beating heart mammary arteries (OPCAB no-touch).
Femoral-iliac cannulation is possible if the ascending aorta is to be avoided or if the patient is too unstable to tolerate induction without this safety feature. Femoral cannulation is also used in reoperations where there is concern about damaging the RA, the RV or the aorta with the sternal saw. Unfortunately, flow through the femoral is often limited. As the flow in the aorta is retrograde, there is a double risk, which can be diagnosed by TEE monitoring.
- Cerebral embolism in extensive atheromatosis of the descending thoracic aorta;
- Retrograde dissection by flow infiltration under atheromatous plaques, which are arranged like roof tiles in the direction of normal blood flow.
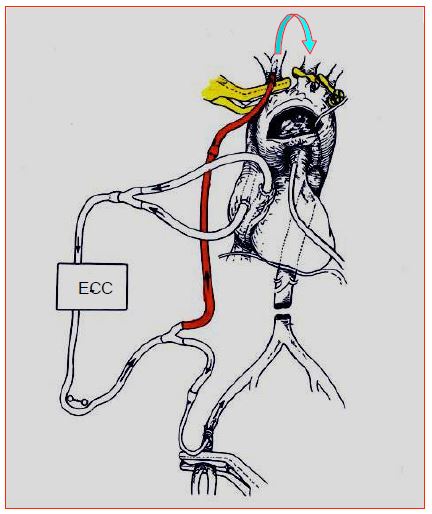
The femoral cannula is occlusive. The lower limb concerned is therefore ischaemic for the duration of the bypass. This situation is tolerable for 3-4 hours. If the cannulation is prolonged, it is prudent to perfuse the limb with a Y-mounted paediatric cannula on the main line and inserted into the distal part of the femoral. A good alternative is the right subclavian artery. This vessel can be cannulated by open puncture or by anastomosing an 8 mm GoreTex™ prosthesis to it in a terminolateral position. The position of the cannula in the aortic arch is monitored on TEE. This system is frequently used for aortic arch surgery, as it allows continuous cerebral perfusion to be maintained (Figure 7.11 and Figure 18.27).
With the brachiocephalic trunk, left carotid and left subclavian arteries clamped at their departure from the arch, the flow perfuses the right carotid and then the left carotid via the circle of Willis and the anastomoses between external carotids. Perfusion pressure (ideally 60 mmHg) is measured in the left radial artery, perfused via collaterals between the left vertebral and subclavian. The flow rate is 10-20 mL/kg/min (1.0-1.5 L/min). The infusion temperature varies from 20° to 32°C depending on the technique. If moderate hypothermia (28-32°C) or normothermia is chosen, cerebral perfusion should be supplemented by continuous subdiaphragmatic perfusion through a femoral cannula to avoid ischaemia of the abdominal viscera, kidneys and marrow.
The filters
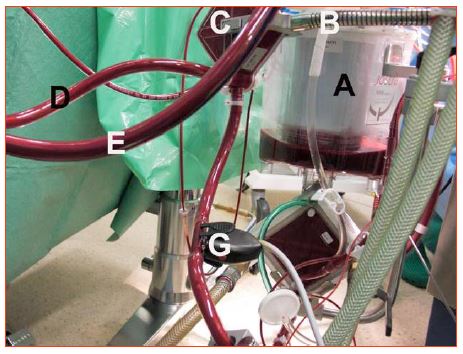
A micropore filter (40 microns) coupled to a "bubble trap" is set up on the arterial circuit, as a last precaution before the patient's aorta. Its purpose is to remove particles that could embolise, and to eliminate any risk of arterial gas embolism. The bubble trap can be opened to room air to prevent the system from becoming pressurised, or continuously purged (200 mL/min), with the blood being returned to the venous reservoir. A filter is also fitted on the venous side (20-40 microns), because the aspirations used in the operating field after the patient has been heparinised return the blood to the venous reservoir. This blood contains cells, debris and procoagulants that must be removed (Figure 7.12).
Figure 7.11: Semi-selective cerebral perfusion through the right subclavian arterial bypass cannula. The brachiocephalic trunk, left carotid and left subclavian arteries are clamped at their origin (yellow). Blood flows from one carotid artery to the other through the anastomoses of the polygon of Willis (arrow).
Figure 7.12: The filters. A: venous reservoir filter. B: return circuit for blood from the patient. C: arterial filter containing the bubble trap; it has a drain hole at the top through which a pump draws blood at low flow and returns it to the venous reservoir. D: arterial circuit leading the blood to the patient. E: venous circuit bringing blood back from the patient. Note the difference in colour between D and E. F: saturometer on the venous circuit. G: saturometer on the arterial circuit.
There are many sources of gas emboli: oxygenator, reservoir, siphon in the circuit, suction by pumps, cardiotomy, cavitation. Changes in blood temperature are always a potential source of gaseous microemboli, as cold dissolved gases shift to gaseous phase when temperature rises [4]. Cavitation is a phenomenon in which microbubbles are created when the local pressure decreases, as in a vortex or upstream of an occlusive pump. Although the evidence is not conclusive, the accumulation of microemboli in organs is probably responsible for some of the postoperative functional damage, particularly neurological. This is why micropore filters are set up in the arterial circuit.
Shunts
Shunt systems must be provided to bypass certain components in case of failure. A shunt is also needed to circulate the priming fluid before the machine is connected to the patient or to avoid blood stagnation when the bypass machine is no longer assisting the patient.
- Arterial filter shunt: maintains arterial perfusion in case the filter becomes blocked, which is rare;
- Distal shunt between arterial and venous cannula: used to circulate priming fluid. It is mandatory in pre-heparinised circuits that operate with ACTs of 300-350 seconds; blood flow cannot be interrupted in these circuits, or clotting may happen in the filters and oxygenator.
| Arterial circuit |
|
The arterial cannula is usually inserted into the ascending aorta, but there are several possible approaches:
- Proximal part of the ascending aorta
- Femoral artery
- Right subclavian artery
The arterial cannula can be the cause of several complications:
- Embolisation of atheromatous plaques
- Sandblasting effect
- Tears
- Dissection
TEE and epiaortic ultrasound can be used to localise plaques and select the safest cannulation technique.
The arterial line is equipped with a particle filter and a bubble trap. It is monitored by a pressure and saturation monitor (SaO2 ).
|
© CHASSOT PG, GRONCHI F, April 2008, last update December 2019
References
- CALAFIORE AM, Di Mauro M, Teodori G, et al. Impact of aortic manipulation on incidence of cerebrovascular accidents after surgical myocardial revascularization. Ann Thorac Surg 2002; 73: 1387-93
- DAVILA-ROMAN VG, BARZILAI B, WAREING TH, et al. Atherosclerosis of the ascending aorta. Stroke 1994; 25:2010-16
- DAVILA-ROMAN VG, BARZILAI B, WAREING TH, et al. Intraoperative ultrasonographic evaluation of the ascending aorta in 100 consecutive patients undergoing cardiac surgery. Circulation 1991; 84(Suppl III):47-53
- GEISSLER HJ, ALLEN JS, MEHLHORN U, et al. Cooling gradients and formation of gazeous microemboli with cardiopulmonary bypass: an echocardiographic study. Ann Thorac Surg 1997; 64:100-8
- KIM KB, KANG CH, CHANG WI, et al. Off-pump coronary artery bypass with complete avoidance of aortic manipulation. Ann Thorac Surg 2002; 74:S1377-82
- KONSTADT SN, REICH DL, KAHN R, et al. Transesophageal echocardiography can be used to screen for ascending aortic atherosclerosis. Anesth Analg 1995; 81:225-33
- MENKIS AH. Management of the ascending aorrta in routine cardiac surgery. Semin Cardiothorac Vasc Anesth 2004; 8:19-24
- MURPHY DA. Recognition and management of ascending aortic dissection complicating cardiac surgical operations. J Thorac Cardiovasc Surg 1983; 85:247-53
- REEVES ST, GLAS KE, ELTZSCHIG H, et al. Guidelines for performing a comprehensive epicardial echocardiographic examination: Recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2007; 20:427-37
- STUMP DA, JONES TJJ, RORIE KD. Neurophysiologic monitoring and outcomes in cardiovascular surgery. J Cardiothorac Vasc Anesth 1999; 13:600-13
- SYLVIRIS S, CALAFIORE P, MATALANIS G, et al. The intraoperative assessment of ascending aortic atheroma: epiaortic imaging is superior to both transesophageal echocardiography and direct palpation. J Cardiothorac Vasc Anesth 1997; 11:704-10