Heparin-induced thrombocytopenia (HIT) is a prothrombotic condition triggered by IgG antibodies to the complex formed between heparin and platelet factor-4 (PF4). This IgG-heparin-PF4 complex activates the platelet, which then releases PF4, ready to form further complexes with heparin. This leads to intense activation of platelets, which release their microparticles and trigger the extrinsic coagulation cascade (tissue factor and factor VIIa) with massive thrombin production. This syndrome has several characteristics [7,14,16].
- Exposure to heparin treatment for 5-10 days; the risk is 1-5% with non-fractionated heparin (UFH) and 0.1-1% with low molecular weight heparins (LMWH); it is 1-5% after heart surgery or trauma [6].
- Thrombocytopenia: a drop in platelet count of at least 50%.
- Venous and arterial thrombosis, with their cascade of complications (35-80% of cases): pulmonary embolism, myocardial infarction, stroke, skin necrosis. In general, venous thrombosis predominates, but arterial thrombosis is more common in cardiac surgery. DIC is present in 10-20% of cases.
- High level of anti-heparin antibodies. This level correlates with thrombotic complications during heparin therapy, but is negative after 3 months because antibodies have a life span of less than 100 days.
The clinical problems are related to thrombin excess, not thrombocytopenia [6]. There are two types of syndrome [7].
- Type I: transient fall in platelets due to a direct effect of heparin on thrombocytes, without clinical significance;
- Type II: massive thrombocytopenic syndrome with a hyperthrombogenic state.
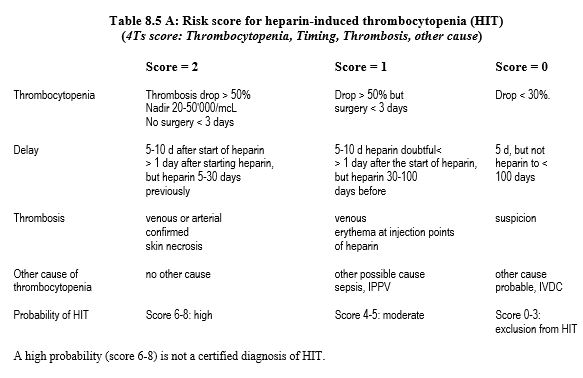
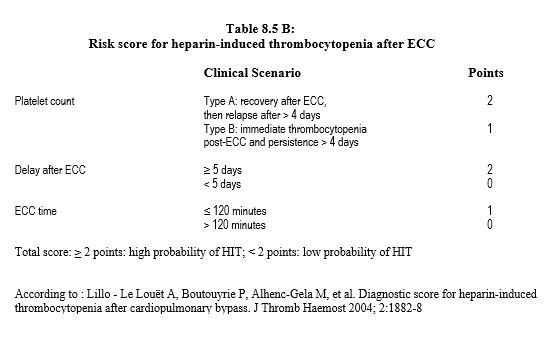
The syndrome is suspected in any patient on heparin who develops thrombocytopenia or thrombosis within 5-15 days of starting treatment. Its probability is assessed by a score that has a high negative predictive value but a low positive predictive value (Table 8.5A) [14]. There is also a score dedicated to after ECC situations (Table 8.5B) [13].
Intermediate and high scores require testing for the presence of heparin-PF4 complex antibodies, which is diagnostic [7,16].
- Immunological tests (ELISA): direct determination of antibodies; the sensitivity of these tests is high, but their specificity is low;
- Functional tests: platelet activation in the presence of heparin and serotonin release; more difficult to perform, these tests are considered gold standard. Heparin UFH should be discontinued at least 4 hours and LMWH at least 12 hours before testing.
Some situations are high risk (> 1%) and require platelet testing every 2-3 days for 2 weeks, particularly on days 5, 7 and 9; these include postoperative heparin administration (incidence in orthopaedics 1-5%), post cardiac surgery (incidence 1-5%) or ventricular assist (incidence 10%), and cancer surgery (incidence 1-2%) [7]. The prevalence is lower in obstetrics and in medical indications for heparin. Women are affected twice often as men [14].
After cardiac surgery, the onset or persistence of thrombocytopenia beyond day 4th is always suspicious, as platelet decline related to ECC, which begins at the end of the pump, normally resolves after4 days. The peak incidence of HIT occurs between 5th and 15th postoperative days. The mortality of the syndrome is 5 to 30%, generally related to thrombosis complications. Their prophylaxis is hampered by the fact that they may occur at the same time as thrombocytopenia [6].
Treatment consists of immediate discontinuation of heparin (UFH and LMWH) and contact with the substance (flushing, heparin-coated catheter). Heparin is replaced by an agent that blocks the factor Xa cascade: argatroban, bivalirudin, desirudin, danaparoide or fondaparinux. Plasmapheresis can remove up to 80% of IgG-heparin-PF4 complexes [7]. Platelet transfusion is only indicated in cases of severe bleeding, but not according to thrombocyte count. Reason being the risk of bleeding is low and platelet transfusion increases the risk of thrombosis. Anticoagulation should be continued for 6 weeks in case of isolated thrombocytopenia and 3 months in ase of associated thrombosis [8]. A vitamin K antagonist (INR 2.0-3.0) is therefore introduced as soon as platelets count normalize (≥ 150,000/mcL), with at least 5 days' recovery from the infused anticoagulant.
Anticoagulants available in place of heparin are not antagonised and are not reversed by protamine. Their elimination depends on their serum half-life (see Direct Thrombin Inhibitors, Table 8.1 and Table 8.2 ) and the use of haemofiltration at the end of ECC [1,4,5,8,16].
Cardiac surgery for HIT
When a patient has heparin-induced thrombocytopenia or a history of heparin-induced thrombocytopenia, there are two possibilities [7,16].
- Anti-heparin antibodies are present:
- If surgery is elective: wait until tests are negative (about 3 months); heparin can then be used for the ECC.
- If surgery is urgent: use alternatives to heparin, i.e. bivalirudin if renal function is normal, argatroban if renal failure. Possible addition: platelet protection.
- Tests for anti-heparin antibodies are negative (< 0.4):
- Use of heparin for ECC.
- In both cases, UFH and LMWH are avoided pre- and postoperatively.
- The tests are repeated 72 hours after heparin administration.
- Bivalirudin (Angiox® ): direct reversible inhibitor of thrombin and platelet activation. Onset of activity: 2-10 minutes; half-life: 25 min (doubled in renal failure); renal elimination. Easiest to handle for ECC, but with risk of thrombosis in the reservoir or oxygenator if machine flow is interrupted, as elimination of bivalirudin by plasma proteolysis continues in the still blood. Therefore, arteriovenous shunts, a CellSaver™ on blood recovery, intermittent flushing of the venous reservoir and storage of the blood in citrated bags are required. The same proteolysis occurs in non-perfused grafts; therefore, cardioplegia should be administered every 15 minutes [7]. Bivalirudin linearly increases ACT, PT, PTT and TT; the most reliable test is the ecarin clotting time (ECT), but this is not available in all laboratories. Dosage: bolus 1 mg/kg + 50 mg in the ECT priming fluid + infusion 2.5 mg/kg/hr. ACT ≥ 450 sec is targeted with additional boluses of 0.1-0.5 mg/kg [10]; target ECT is 400-500 sec [16]. The infusion is stopped 10-15 minutes before the end of the ECC. Ultrafiltration is only used after ECC to accelerate the elimination of bivalirudin.
- Argatroban (Argatroban Injection® ): synthetic molecule that selectively and reversibly binds to thrombin. Half-life: 40-50 minutes; hepatic elimination. Dosage: bolus 0.1-0.2 mg/kg iv + 0.05 mg/kg in ECC priming fluid + infusion 5-10 mcg/kg/min (immediate and continuous) for ACT 350-400 sec and aPTT 1.5 to 3 times baseline; additional boluses if necessary: 2 mg Return to normal coagulation 2-4 hoursaftercessation of infusion. Argatroban is the drug of choice in cases of renal dysfunction.
- Danaparoid sodium (Orgaran® ): Predominantly inhibits factor Xa. Half-life of 7 hours for anti-IIa activity and 25 hours for anti-Xa activity; renal elimination. Dosage: bolus iv 1,500-2,000 U + 5,000-10,000 U in the ECC priming fluid; addition of 1,500 U after 2 hours. Very difficult to manage on ECC and very haemorrhagic postoperatively.
- Lepirudin (Refludan® ): recombinant form of hirudin, irreversible thrombin inhibitor discovered in leeches. Serum half-life 10 min, elimination half-life 1-1.5 hours, but irreversible thrombin inhibition; renal elimination. Best monitored by ECT (ecarin clotting time of citrated whole blood). Dosage: bolus 0.25 mg/kg + 0.2 mg/kg in the ECC priming fluid + bolus 5 mg for ACT > 350 sec (imprecise); infusion 0.15 mg/kg/h. Lepirudin production ceased in 2012.
Bivalirudin, the only substance tested in controlled studies, is currently considered the first choice to replace heparin in cardiac surgery [9,10,14]. Two other substances are available for use in HIT, but are not suitable for use in ECC because of their subcutaneous administration.
- Desirudin (Iprivask® ): recombinant form of hirudin. Elimination half-life: 2-3 hours. Suggested dosage for HIT: 15-30 mg/12h subcutaneous according to aPTT, but experience with this substance is very limited.
- Fondaparinux (Arixtra® ): synthetic analogue of the pentasaccharide sequence of heparin, selective inhibition of factor Xa. Half-life: 16-17 hours; excreted by the kidneys, it should be avoided in renal failure or halved if creatinine clearance is 30-50 mL/min. Dosage: 5 mg/day subcutaneously (10 mg/d > 100 kg). However, the efficacy of fondaparinux is not based on any randomised study; the drug has even been suspected of triggering HIT [16].
- Oral anti-Xa anticoagulants (rivaroxaban, apixaban, edoxaban): under investigation.
Platelet inhibition (thromboplegia) is an additional option to anticoagulation. It consists of anticoagulating patients with heparin, and in parallel blocking platelet aggregation induced by the action of pre-formed IgG antibodies on PF4-heparin complexes by means of a short-acting antiplatelet drug. Although still responsive to heparin, patients are no longer at excessive risk of thromboembolism or thrombocytopenia [15].
- Heparin (usual dose) + prostacyclin (Iloprost® ). Half-life 15-30 min. Infusion of 3-12 ng/kg/min (avg 7.6 ng/kg/min), set according to HIPA (Heparin-induced platelet aggregation) test for < 5% platelet activation. The infusion is halved after protamine and reduced to one quarter on arrival in the ICU and stopped 1 hour later [15]. Disadvantages: iloprost causes hypotension (regulated by norepinephrine), and intraoperative HIPA tests are time consuming (30-40 minutes).
- Heparin (bolus 5,000 IU and infusion 1,000 IU/h) + tirofiban (Aggrastat® ): blocker of the GP IIb/IIIa receptor of platelets, responsible for the binding of platelets with fibrinogen (see Figures 8.12 and 8.13). Half-life: 2 hours. Bolus 0.4 mcg/kg, then infuse 0.15 mcg/kg/hour. Stop 1 hour before the end of the ECC [11].
- Heparin (usual dose) + cangrelor (Kengrexal® ): reversible direct P2Y receptor inhibitor12 marketed for PCI and acute coronary syndrome, still in clinical trial for this indication. Half-life 9 minutes. Infusion 0.75 mcg/kg/min [2,12]. Interruption 30 minutes before protamine.
| Heparin-induced thrombocytopenia (HIT) |
|
HIT (heparin-induced thrombocytopenia) is triggered by antibodies to the complex formed by heparin and platelet factor-4 (PF4), resulting in activation/consumption of thrombocytes and initiation of the coagulation chain. Characteristics :
- Heparin treatment for 5-10 days
- Thrombocytopenia (fall > 50%)
- Venous and arterial thrombosis
- High level of anti-heparin antibodies (attenuated beyond 3 months)
- Incidence: 1-5% with UFH, 0.1-1% with LMWH, more frequent after surgery
- Mortality: 5-10%.
Treatment: discontinue heparin and replace with a subcutaneous alternative (long-term treatment):
- Desirudine (Iprivask® )
- Fondaparinux (Arixtra® )
Substances for use in case of ECC in the event of HIT :
- Bivalirudin (Angiox® ), easy to handle
- Argatroban (Argatroban Injection® ), preferable in cases of renal failure
|
© CHASSOT PG, MARCUCCI Carlo, last update November 2019.
References
- ADAMS RLC, BIRD RJ. Review article: Coagulation cascade and therapeutic update: Relevance to nephrology. Part I: Overview of coagulation, thrombophilia and history of anticoagulants. Nephrol 2009; 14:462-70
- ANGIOLILO DJ, FIRSTENBERG MS, PRICE MJ, et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery. JAMA 2012; 307:265-74
- ANTONIOU T, KAPETANAKIS EI, THEODORAKI K, et al. Cardiac surgery in patients with heparin-induced thrombocytopenia using preoperatively determined dosages of Iloprost. Heart Surg Forum 2002; 5:354-7
- COPPENS M, EIKELBOOM JW, GUSTAFSSON D, WEITZ JI. Translational success stories. Development of direct thrombin inhibitors. Circ Res 2012; 111:920-9
- GARCIA DA, BAGLIN TP, WEITZ JI, et al. Parenteral anticoagulants: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e24S-e43S
- GREINACHER A. Heparin-induced thrombocytopenia. N Engl J Med 2015; 373: 252-61
- IVASCU NS, FITZGERALD M, GHADIMI K, et al. Heparin-induced thrombocytopenia: a review for cardiac anesthesiologists and intensivists. J Cardiothorac Vasc Anesth 2019; 33: 511-20
- KELTON JG, ARNOLD DM, BATES SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med 2013; 368:737-44
- KOSTER A, BUZ S, et al. Bivalirudin anticoagulation during cardiac surgery: a single-center experience in 141 patients. Perfusion 2009; 24: 7-11
- KOSTER A, DYKE CM, ALDEA G, et al. Bivalirudin during cardiopulmonary bypass in patients with previous or acute heparin-induced thrombocytopenia and heparin antibodies: Results of the CHOOSE-ON trial. Ann Thorac Surg 2007; 83:572-7
- KOSTER A, KUKUCKA M, BACH F, et al. Anticoagulation during cardiopulmonary bypass in patients with heparin-induced thrombocytopenia type II and renal impairment using heparin and the platelet glycoprotein Iib/IIIa antagonist tirofiban. Anesthesiology 2001; 94:245-51
- KRAJEWSKI S, KURZ J, NEUMANN B, et al. Short-acting P2Y12 blockade to reduce platelet dysfunction and coagulopathy during experimental extracorporeal circulation and hypothermia. Br J Anaesth 2012; 108:912-21
- LILLO LE LOUËT A, BOUTOUYRIE P, ALHENC-GELA M, et al. Diagnostic score for heparin-induced thrombocytopeniaaftercardiopulmonary bypass. J Thromb Haemost 2004; 2:1882-8
- LINKINS LA, DANS AL, MOORES LK, et al. Treatment and prevention of heparin-induced thrombocytopenia. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (Suppl 2):e495S-e530S
- PALATIANOS G, MICHALIS A, ALIVIZATOS P, et al. Perioperative use of iloprost in cardiac surgery patients diagnosed with heparin-induced thrombocytopenia-reactive antibodies or with true HIT (HIT-reactive antibodies plus thrombocytopenia): an 11-year experience. Am J Hematol 2015; 90:608-17
- SALTER BS, WEINER MM, TRINH MA, et al. Heparin-induced thrombocytopenia. A comprehensive clinical review. J Am Coll Cardiol 2016; 67:2519-32