The different types of shunt are: right → left (cyanotic), left → right (non-cyanotic), bidirectional, and therapeutic (for improving pulmonary blood flow). Their flow rate is proportionate to the diameter of the orifice (or conduit) and the impedance ratio between the upstream and downstream chambers. They increase if the upstream pressure rises or the downstream pressure falls, and decrease if the upstream pressure falls or the downstream pressure rises. A shunt's flow rate can therefore be modified by adjusting systemic vascular resistance (SVR) and pulmonary vascular resistance (PVR) [5].
- R-to-L shunt (tetralogy of Fallot, VSD + pulmonary atresia, TGA, single ventricle, Fontan) (Figure 15.61) (Table 15.4): the shunt is increased if SVR falls or PVR rises. Cyanosis is proportional to the drop in systemic pressure. The aim is therefore to maintain high SVR and low PVR. SpO2 fluctuates in line with the Qp/Qs ratio. An increase in FiO2 has a negligible effect on cyanosis linked to this type of shunt. Direct flow from the systemic venous return to the arterial line accelerates induction with intravenous agents. In contrast, uptake and elimination of halogenated agents are slowed due to low pulmonary blood flow [2]. ETCO2 gives an underestimation of actual PaCO2 due to the dead-space effect caused by blood volume bypassing the lungs [4].

Figure 15.61: Right → left shunt. This is increased when SVR falls, which lowers pulmonary arterial blood flow (Qp). The shunt is reduced if SVR increases and PVR falls. As a result, pulmonary flow increases and SaO2 improves.
- L-to-R shunt (ASD, AVC, VSD, patent ductus arteriosus) (Figure 15.62) (Table 15.3): it is reduced if SVR falls or PVR rises. The aim is rather to reduce systemic pressure. The major issue is often the presence of pulmonary hypertension. High SpO2 levels do not necessarily indicate that O2 delivery (DO2) is satisfactory. When the Qp/Qs ratio ≥ 3:1, DO2 falls even if the arterial blood is sufficiently oxygenated [1]. Induction with intravenous agents is slowed due to the dilution of venous blood by arterialised blood. Uptake and elimination of halogenated agents are theoretically accelerated by high pulmonary blood flow, although the clinical effect is negligible [3].

Figure 15.62: Left → right shunt. This is increased if SVR rises and PVR falls, in which case pulmonary blood flow (Qp) is excessive. The shunt is reduced if SVR falls and PVR rises. This is the goal pursued by anaesthesia. Since LV compliance decreases with age, LV end-diastolic pressure tends to increase, raising LA pressure and reinforcing L-to-R shunting.
- L-to-R palliative shunt (Blalock-Taussig, Waterston or Potts central shunt, aortopulmonary collateralisation) (Videos and Figure 15.63 and Figure 15.64): the flow rate is determined by the conduit diameter and may result in left ventricular overload. Pulmonary blood flow is directly proportional to systemic pressure. SpO2 therefore decreases with SVR. Systemic hypotension therefore has catastrophic effects on arterial oxygen saturation. An alpha-stimulant (phenylephrine, norepinephrin) is required in the event of arterial desaturation. Diastolic pressure is low (PAP < systemic AP during diastole) and may compromise the coronary circulation.
Video: Continuous systolo-diastolic flow in the left subclavian artery in a case of Blalock shunt (short-axis view of the distal aortic arch).
Video: Continuous systolo-diastolic flow in a Blalock shunt between the subclavian artery and the pulmonary artery.
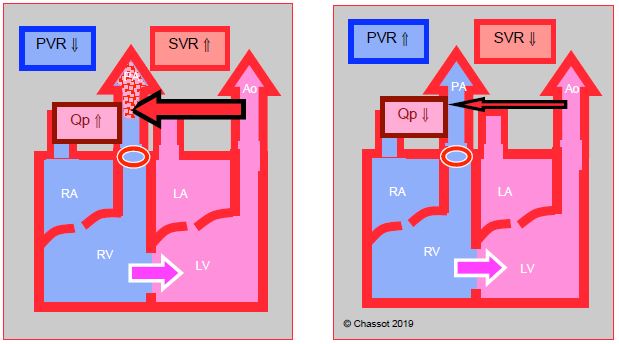
Figure 15.63: Left → right corrective shunt to increase pulmonary blood flow (Qp) in the event of pulmonary stenosis. Its blood flow decreases as SVR falls and also rises as SVR increases.

Figure 15.64: Blalock-Taussig shunt. A: flow in the subclavian artery before the shunt is constructed. The flow is solely systolic as in any systemic artery. B: after connection to the pulmonary artery, the flow becomes systolic-diastolic as in a L→R shunt.
The aim of managing patients with shunts is to balance the pulmonary blood flow (Qp) and systemic blood flow (Qs) and achieve a Qp/Qs ratio close to 1:1. To that end, PVR and SVR are manipulated to lower Qp in a L-to-R shunt (high PVR and low SVR) and increase Qp in a R-to-L shunt (low PVR and high SVR), with a view to achieving the best SaO2 without metabolic acidosis. Moreover, shunts potentiate the effects of hypovolaemia on arterial pressure due to the constant loss of volume in the low-pressure pulmonary circuit.


| Shunts and anaesthesia |
| R-to-L shunt: maintain high SVR and low PVR to reduce cyanotic shunting - IV induction accelerated - Inhalation induction/recovery slowed L-to-R shunt: maintain low SVR and high PVR to reduce shunting (non-cyanotic) - IV induction slowed - Inhalation induction/recovery accelerated Palliative L-to-R shunt: maintain high SVR to improve pulmonary flow |
© BETTEX D, CHASSOT PG, January 2008, last update May 2018
References
- HEGGIE J, POIRER N, WILLIAMS WG, KARSKI J. Anesthetic considerations for adult cardiac surgery patients with congenital heart disease. Sem Cardiothorac Vasc Anesth 2003; 7:141-52
- HUNTINGTON JH, MALVIYA S, VOEPEL-LEWIS T, et al. The effect of a right-to-left intracardiac shunt on the rate of rise of arterial and end-tidal halothane in children. Anesth Analg 1999; 88:759-62
- LAIRD TH, STAYER SA, RIVENES SM, et al. Pulmonary-to-systemic blood flow ratio effects of sevoflurane, isoflurane, halothane and fentanyl/midazolam with 100% oxygen in children with congenital heart disease. Anesth Analg 2002; 95:1200-6
- PERLOFF JK. Survival patterns without cardiac surgery or interventional catheterization: a narrowing base. In: PERLOFF JK, CHILD JS, Eds. Congenital heart disease in adults. 2nd edition. Philadelphia: WB Saunders, 1998, 15-53
- WARNES CA, WILLIAMS RG, BASHORE TM, et al. ACC/AHA 2008 Guidelines for the management of adults with congenital heart disease: executive summary. Circulation 2008; 118:2395-451
