Transoesophageal echocardiography (TEE) plays a key role in valve surgery as it has a very significant impact [1,7,8].
- It guides the anaesthetist and surgeon to the best technique to ensure haemodynamic balance and optimise the surgical procedure. In valve surgery, the rate of change in the treatment plan based on intraoperative TEE is 11-14%, compared with an average of 7% in all cardiac surgery [2,4,7,9]. In addition, in 6-8% of cases, TEE revealed a cardiac lesion that had not been suspected at preoperative assessment, justifying an additional unplanned correction [5,13].
- Prior to ECC, the functional anatomy of the heart can be assessed, i.e. how the structures have adapted to the valvular disease and the degree of decompensation of the myocardium (see Figure 11.14). In the case of polyvalvular disease, this examination makes it possible to assess the predominant lesions and helps to define the primary pathology and the secondary or functional lesions.
- After ECC, it allows immediate monitoring of the quality of valve reconstruction and prosthesis function, highlighting any residual defects. In 2-6% of cases, it justifies a return to bypass surgery to correct an anomaly (paravalvular leak, inadequate prosthesis) [2,4,5,7]; this repeat operation also avoids a subsequent reoperation with a higher mortality rate.
- For anaesthetists, TEE offers unrivalled monitoring of blood volume in situations where filling pressures are poor reference points due to diastolic dysfunction and valvular disease. It provides a highly accurate analysis of ventricular function despite caval remodelling and variations in stress and systolic performance during procedures. It can be used to diagnose haemodynamic effects such as dynamic stenosis of the LV outflow tract that cannot be detected by other methods.
- TEE is an integral part of certain procedures, as this imaging is required for the placement of certain cannulae or prostheses and for immediate visualisation of the surgical procedure (implantation of stentless or endovascular prostheses, minimally invasive surgery, percutaneous plasty, etc.).
Intraoperative TEE is a definite recommendation for valve surgery, for the insertion of an unmounted prosthesis or homograft, and in cases of endocarditis; it is desirable, but not essential, for the insertion of a mechanical prosthesis or a mounted bioprosthesis [10,15].
The examination must always be complete: morphology and function of both ventricles, valve anatomy, colour flow and spectral Doppler, calculation of gradients and orifice areas. Any discrepancies between the various elements must be explained. As flow measurements are dynamic, they are dependent on the patient's circulatory state, which is significantly altered by anaesthesia. For example, inadequacy decreases by 1 to 2 degrees on a scale of 4 simply as a result of general anaesthesia [6]. Any quantification of regurgitation must be made after normal haemodynamics have been restored, if necessary with an arterial vasopressor. If the patient is arrhythmic, measurements should be taken over cardiac cycles with a diastolic duration corresponding to a frequency of approximately 70 beats/minute, repeated and averaged over 3-5 measurements.
After valve replacement, TEE is essential to assess the reconstruction: tightness, residual prolapse, restriction to anterograde flow, dynamic obstruction of the LVOT, etc. Residual leak must be tested under severe haemodynamic conditions (MAP 90-100 mmHg). Residual leak must be tested under severe haemodynamic conditions (MAP 90-100 mmHg). Immediate monitoring after mitral valve surgery has excellent predictive value in the medium term, as there is no significant change in the degree of regurgitation between that found at the end of ECC and that diagnosed six months after surgery [3,12]. After prosthetic valve implantation, TEE should answer several questions:
- Is the amount of leakage normal?
- Is there a paravalvular leak?
- Is the orifice free and does the occlusion show normal coaptation?
- Are the flows and gradients within the norms for the type of prosthesis?
- What is the effect of replacing one valve on the function of the others? Is it necessary to work on other valves?
- Is there a dynamic obstruction in the atrial chamber?
- Is the ventricular function adequate?
- Have there been changes in segmental kinetics?
- The right and left upper pulmonary veins;
- The upper part of the left interatrial septum;
- The mitral valve angle;
- The apical septum of the left ventricle (in Trendelenburg position);
- The right aortic sinus of Valsalva;
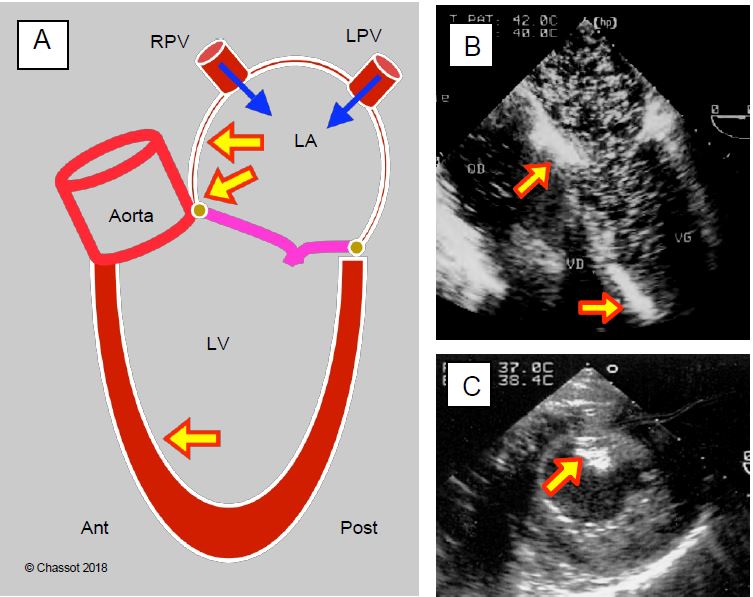
- To assess the extent of systemic emboli, bubbles can be observed in the descending aorta and the TEE probe can be rotated backwards.
- Continuous suction maintained by the cardioplegia cannula in place at the root of the ascending aorta;
- Shaking and manipulation of the heart;
- Rolling the operating table;
- Trendelenburg position;
- Hyperinflation and lung compression;
- Inotropic stimulation;
- Atrial drainage by direct needle puncture and aspiration;
- Ventricular drainage by apical or transseptal puncture.
| Intraoperative use of TEE |
|
The value of TEE in valve surgery
- Assessment of blood volume and ventricular function independent of compliance and remodelling.
- Modification of surgical strategy based on pre-ECC TEE in 6-8% of cases
- Immediate verification of valve reconstruction or prosthesis function
(return to ECC in 2-6% of cases)
- Real-time visualisation of the surgical procedure in the case of valve implantation or percutaneous procedures
- Debubbling of the left cavities
- Immediate identification of surgical complications
- Diagnosis of dynamic obstruction of the irrigation chamber
|
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- CHALIKI HP, CLICK RL, ABEL MD. Comparison of intraoperative transesophageal echocardiographic examination with the operative findings: prospective review of 1918 cases. J Am Soc Echocardiogr 1999; 12:237-40
- CLICK RL, ABEL MD, SCHAFF HV. Intraoperative transesophageal echocardiography: 5-year prospective review of impact on surgical management. Mayo Clin Proc 2000; 75:241-7
- CZER LSC, MAURER G. Intraoperative echocardiography in mitral and tricuspid valve repair. Echocardiography 1990; 7:305-12
- DESJARDINS G, CAHALAN M. The impact of routine trans-oesophageal echocardiography (TOE) in cardiac surgery. Best Pract Res Clin Anaesthesiol 2009; 23:263-71
- ELTZSCHIG HK, Rosenberger p, löffler m, et al. Impact of intraoperative transesophageal echocardiography on surgical decisions in 12,566 patients undergoing cardiac surgery. Ann Thorac Surg 2008;85 :845-52.
- GREWAL KS, MALKOWSKI MJ, PIRACHA AR, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesoophageal echocardiography. Am J Cardiol 2000; 85:199-203.
- MICHELENA HI, ABEL MD, SURI RM, et al. Intraoperative echocardiography in valvular heart disease: An evidence-based appraisal. Mayo Clin Proc 2010; 85:646-55
- MICHEL-CHERQUI M, FISCHLER M. Intraoperative echocardiography in cardiac surgery. In: VIGNON P et al, eds. Echocardiographie Doppler chez le patient en état critique. Paris: Elsevier-Masson, 2008, 433-80
- MISHRA M, CHAUHAN R, SHARMA KK, et al. Real-time intraoperative transesophageal echocardiography - How useful? Experience of 5'016 cases. J Cardiothorac Vasc Anesth 1998; 12:625-32
- NISHIMURA RA, OTTO CM, BONOW RO, et al. 2014 AHA/ACC Guideline for the management of patients with valvular heart disease. Circulation 2014; 129:e521-e643
- ORIHASHI K, HONG Y, KEEHN L, et al. Location of retained intracardiac air in coronary artery bypass grafting using transesophageal echocardiography. Anesthesiology 1990; 73(3A):A430
- REICHERT SLA, VISSER CA, MOULIJN AC, et al. Intraoperative transesophageal color-coded Doppler for evaluation of residual regurgitation after mitral valve repair. J Thorac Cardiovasc Surg 1990; 100:756-63
- SUTTON DC, KLUGER R. Intraoperative transoesophageal echocardiography: Impact on adult cardiac surgery. Anaesth Intensive Care 1998; 26:287-93
- TINGLEFF J, JOYCE F, PETTERSON G. Intraoperative echocardiographic study of air embolism during cardiac operations. Ann Thorac Surg 1995; 60:673-7.
- VAHANIAN A, ALFIERI O, ANDREOTTI F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012; 33:2451-96
