- Preload: Total blood volume is increased to maintain efficient systemic flow in the face of volume loss between the LV and LA. Hypovolaemia reduces systemic flow proportionally more than the regurgitant fraction, as the atrial leak constantly "steals" volume from the low pressure system of the LA. Tolerance to hypovolemia is low and preload must be maintained normal to high.
- Afterload: As the LV functions as a cavity with two outlets, the volume regurgitated is a direct function of the resistance to ejection and the SAR must therefore be kept low. Arterial vasodilatation is necessary (isoflurane, nitroprusside, phentolamine).
- Contractility: must be kept high to ensure anterograde systolic flow. In chronic MI, low afterload and high preload mask the true contractility of the LV, which is poorly reflected by the usual indices of function. Therefore, systolic dysfunction should be suspected and only inotropic agents without alpha effects should be used (dobutamine, amrinone, milrinone, levosimendan). In cases of difficulty, intra-aortic balloon pump is highly effective in reducing LV pressure, reducing MI and improving coronary perfusion.
- Rate: this must be maintained as bradycardia increases filling time and ventricular volume, with the risk of LV dilatation. As MI occurs in systole, changes in rate have little effect on its duration. Maintaining sinus rhythm is important if the LV is not very dilated; in this case, its contractility is low and the volume driven by its contraction is negligible.
- Pulmonary resistance: Pulmonary hypertension is common but generally moderate; the pulmonary vascular musculature is hyperreactive. Avoid any pulmonary vasoconstriction (hypoxia, hypercarbia, acidosis, N2O) and maintain hypercapnia with a pH of 7.5 and a PaCO2 of 32-35 mmHg.
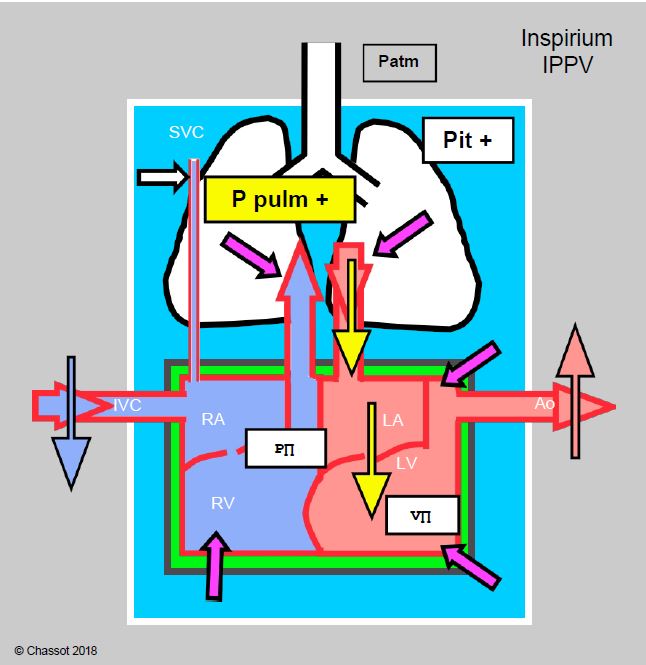
- Positive pressure ventilation: this improves left-sided flow as long as venous return to the right heart is assured. Lung emptying into the LA is accelerated, anterograde mitral flow is increased and atrial transmural pressure is reduced; effective LV afterload is reduced (see Figure 11.97 and Chapter 5 Cardiorespiratory Interactions, Positive Pressure Ventilation). PPV therefore tends to reduce MI; its effects are mediated via mean intrathoracic pressure.
Figure 11.97: Cardiorespiratory interactions of positive pressure ventilation (PPV) in mitral valve disease. Schematic representation of the effects of the increase in intrathoracic pressure (Pit) due to inspiratory IPPV and PEEP. The increase in Pit and intrapulmonary pressure (purple arrows) increases RV afterload, LA filling and LA preload, compresses the ventricles, slows venous return from the inferior vena cava (IVC) and facilitates LV ejection because its afterload does not change (it is outside the chest). As long as right heart flow is compensated, the situation is better than with spontaneous ventilation because anterograde flow through the mitral valve is facilitated and pulmonary congestion is reduced.
Intraoperative transesophageal echocardiography is essential to elucidate the mechanisms of MI and to plan the management strategy [2]. However, the assessment of MI is altered by anaesthesia, which tends to reduce its significance by reducing blood pressure and filling. MI is reduced by 25-40% in half of patients simply because they are asleep [1,3]. Any quantification of MI should be performed after restoration of normal haemodynamics (MAP ≥ 80 mmHg), if necessary with vasopressors.
The Swan-Ganz pulmonary catheter is very useful in all mitral operations to regulate fluid administration, to monitor the evolution of pressures in the LA and to assess the anterograde systolic volume (SV obtained by pulmonary thermodilution); the SvO2 makes it possible to assess the adequacy of systemic flow to the body's needs. On the PCWP curve, the size of the "v" wave is not directly proportional to that of the MI because it depends on atrial compliance; when the atrium is dilated, the LA absorbs the pressure surge and buffers the "v" wave. Flow measurements may be biased in the presence of significant secondary tricuspid regurgitation. After ECC, correction of mitral regurgitation imposes difficult conditions on the LV: increased afterload, reduced preload. In these conditions, the Swan-Ganz catheter is very useful for the management of haemodynamic support.
Anaesthetic technique for mitral valve surgery in MI
- Induction can be achieved with several agents depending on the degree of left ventricular dysfunction and pulmonary hypertension:
- Etomidate: the safest agent, without haemodynamic effects;
- Midazolam: beneficial reduction of sympathetic tone, but slow induction and emergence;
- Propofol: reduced pre- and, to a lesser extent, post-load; suitable in reduced doses and administered slowly in non-decompensated cases;
- Addition of fentanyl or sufentanil to reduce sympathetic response;
- Topical laryngeal and tracheal anaesthesia should be used to prevent hypertension during intubation;
- Thiopental, ketamine: not recommended due to negative inotropic effect (thiopental, ketamine) and sympathetic stimulation with increased SAR (ketamine).
- Hypertension is more dangerous than hypotension.
- Maintenance of anaesthesia:
- Isoflurane preferred ( ↓ SAR); sevoflurane adequate;
- Propofol perfusion;
- Desflurane: not recommended (SAR and PAR);
- PPV + PEEP beneficial.
- Keep SARs low, avoid high blood pressure (pain, alertness).
- Maintain circulatory volume (perfusion) and avoid drop in preload (avoid hypovolaemia).
- Maintain contractility (deterioration of systolic function is masked by MI):
- Dobutamine preferred; dopamine adequate if dose < 5 mcg/kg/min;
- Milrinone (± adrenaline) in PHT, dilation or chronic LV failure.
- Avoid bradycardia (risk of LV dilatation); tachycardia is not dangerous.
- In left heart failure, IABP is highly effective in reducing MI.
- TEE monitoring:
- Mechanisms and variations of MI;
- LV size and function, RV function;
- Assessment of blood volume;
- Post-ECC: assessment of plasty, diagnosis of SAM, LV function.
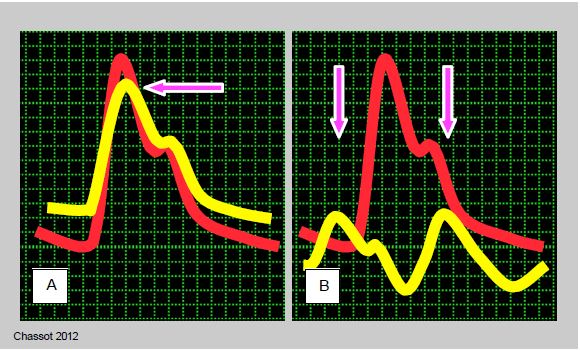
- Pulmonary artery catheter: more useful after bypass than before because of postoperative LV dysfunction. When positioning in Pcap, the PAP curve should be superimposed on the systemic BP curve in order to distinguish the PAPsyst and the giant "v" wave by their synchronisation with the peak of the systemic BP, since their values can be very close; the PAPsyst is synchronous with the peak of the PAsyst, whereas the "v" wave is synchronous with the arterial dicrotism (see Figure 6.21).
- PAP in PHT;
- PCWP (adjustment of infusions);
- Measurement of anterograde SV (effective SV) and SvO2 (adequacy of CO to metabolic needs);
- SV and CO after ECC; measurement of SAR.
- ScO2: assessment of effective peripheral perfusion.
- CO measurement by arterial curve analysis (PiCCO™) can replace Swan-Ganz as long as data such as total lung water and global preload are used and the system is regularly recalibrated by transpulmonary thermodilution. However, it does not provide information on PAP or PCWP.
Figure 6.21: Distinguishing between the pulmonary artery (PA) and pulmonary artery occlusion (PCWP) curves can sometimes be difficult, especially when the "v" wave is very large in massive mitral regurgitation. By superimposing the Swan-Ganz curve (yellow) on the systemic arterial catheter curve (red), the difference becomes clearer: while the PA pressure peak is synchronous with that of the systemic artery, the "a" wave occurs before that of the arterial pressure and the "v" wave occurs at the time of arterial dicrotism. A: Superimposition of the systemic arterial curve and the PA curve. B: Overlay of the systemic arterial curve and the PCWP curve. The pressure scales range from 0 to 120 mmHg for the systemic artery and from 0 to 30 mmHg for the Swan-Ganz.
| Hemodynamics sought in mitral insufficiency |
|
Normal to high blood volume Systemic vasodilation Non-alpha inotropic stimulation (amines β , inodilators) Normal to high frequency Pulmonary vasodilation in PHT Beneficial positive ventilation Full - Tonic - Open |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- GREWAL KS, MALKOWSKI MJ, PIRACHA AR, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesoophageal echocardiography. Am J Cardiol 2000; 85:199-203.
- MICHELENA HI, ABEL MD, SURI RM, et al. Intraoperative echocardiography in valvular heart disease: An evidence-based appraisal. Mayo Clin Proc 2010; 85:646-55
- SANFILIPPO F, JOHNSON C, BELLAVIA D, et sl. Mitral regurgitation grading in the operating room: a systematic review and meta-analysisi comparing preoperative and intraoperative assessments during cardiac surgery. J Cardiothorac Vasc Anesth 2017; 31:1681-91