Angio-CT
The advantage of multi-slice CT is that it can highlight calcified areas and even calculate an index of the degree of calcification, making it possible to quantify calcific invasion of the aortic valve in degenerative stenosis or the mitral annulus in mitropathy. In aortic stenosis, valve calcification is an independent predictor of perioperative mortality (HR 1.71) [3]. Its high spatial resolution makes it an essential examination for imaging the anatomical structures of the aortic valve prior to transcatheter aortic valve implantation (TAVI, see Figure 10.25). It can also be used to perform non-invasive coronary angiography, the accuracy and high negative predictive value of which are sufficient to exclude coronary intervention when appropriate. Angio-CT provides excellent visualisation of the entire thoracic aorta and the vessels of the aortic arch, allowing precise measurements of diameter, wall thickness and areas of calcification.
MRI
MRI is the gold standard for measuring ventricular volumes, particularly the RV volume of, whose crescent shape around the LV precludes any reliable geometric approximation. MRI is also invaluable for assessing tissue structure, particularly the presence of myocardial fibrosis (late gadolinium enhancement) [2]. Its spatial resolution is inferior to that of CT.

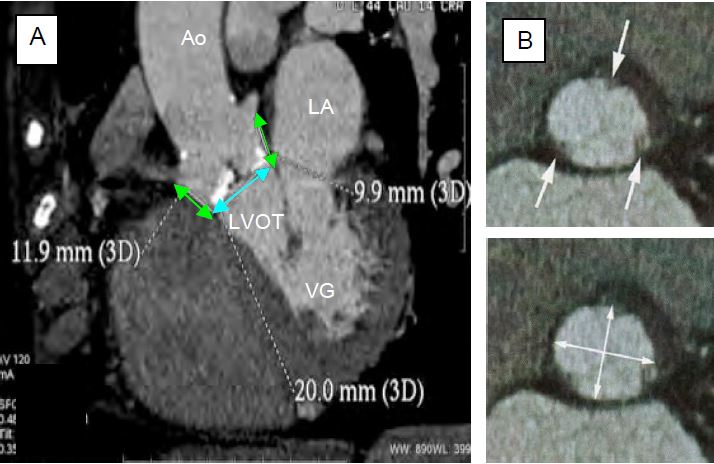
Figure 10.25: Pre-operative CT scan measurements of the aortic root. A: CT scan; measurement of the aortic annulus (blue arrow) and the distance to the coronary ostia (green arrows); in white: calcified areas. B: CT scan of the aortic annulus; the arrows indicate where the cusps intersect the annulus. Bottom: Measurement of the ellipse diameter [Achenbach S, et al. J Cardiovasc Comput Tomogr 2012; 6:366-80. Ferrari E, et al. Ann Thorac Surg 2010; 89:1925-32].
Fluoroscopic cine angiography
This is the only test that provides a satisfactory assessment of the opening and closing kinetics of the leaflets in a mechanical prosthetic valve.
Catheterisation
Cardiac catheterisation is now only used when the other tests are so contradictory that an operative decision cannot be made [1].
Knowing the cardiac output using Fick's formula or thermodilution, the surface area of a stenotic valve can be calculated using the simplified Gorlin formula, as the flow rate and pressure gradient across an orifice are geometrically related [4,5]:
MSA (cm2) = K · CO / √ΔPm
where:
CO = cardiac output (L/min)
ΔPm = mean pressure gradient (mmHg)
K = constant
From this it can be deduced that the transvalvular pressure gradient is a function of the square of the flow velocity (ΔP ≈ V2 ), irrespective of the surface area of the valve.
The transthoracic gradient measured by catheterisation is not the same as that measured by echo, as there are three different gradients (see Figure 11.28B) [6].
- Peak-to-peak gradient (catheterisation): difference between the maximum ventricular and aortic pressures; these two pressures are not simultaneous because the stenosis causes a delay in the rise of the aortic pressure (pulsus tardus). This gradient does not exist in nature.
- Maximum gradient (echo): the pressure difference calculated at the highest transvalvular velocity occurring during the acceleration of the flow at the beginning of systole (maximum instantaneous gradient). This gradient is not affected by the pressure recovery phenomenon.
- Mean gradient (echo): average of the sum of instantaneous gradients; the mean gradient is a better estimate of the degree of obstruction because it is less dependent on haemodynamic conditions and less prone to overestimation of stenosis.
The peak-to-peak gradient is significantly smaller than the maximum gradient. When peak-to-peak gradient is measured in the operating theatre, the LV outflow tract is usually punctured for upstream pressure, but this should not be compared with femoral or radial pressure, which is physiologically amplified in the periphery; the calculation should be made with the aortic pressure measured by fine needle puncture as close to the valve as possible [6].
Coronary angiography
Coronary angiography is indicated prior to valvular heart surgery in certain situations where simultaneous myocardial revascularisation is highly likely, as angio-CT is only useful to rule out coronary disease [7].
- History of angina and/or myocardial infarction;
- Changes in LV segmental kinetics;
- Age > 45 in men and postmenopausal in women;
- Presence of cardiovascular risk factors;
- Moderate to severe mitral regurgitation, secondary and probably ischaemic in nature.
Coronary artery stenosis ≥ 70% is an indication for coronary artery bypass grafting at the same time as mitral or aortic surgery.
| Other examination techniques |
| Angio-CT: excellent spatial resolution, quantification of calcium deposits, possibility of triage coronary angiography. MRI: reference for the calculation of ventricular volumes (in particular RV), assessment of the myocardium (fibrosis, viability). Cine angiography: movement of the leaflets of prosthetic valves. Catheterisation: only used when data from other tests are too inconsistent Coronary angiography: if coronary revascularisation is being performed at the same time. |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BAUMGARTNER H, FALK V, BAX JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739-86
- BAX JJ, DELGADO V. Advanced imaging in valvular heart disease. Nat Rev Cardiol 2017; 14:209-23
- CLAVEL MA, PIBAROT P, MESSIKA-ZEITOUN D, et al. Impact of aortic valve calcification as measured by MDCT, on survival in patients with aortic stenosis; results of an international registry study. J Am Coll Cardiol 2014; 64:1202-13
- GORLIN R, GORLIN SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and the central circulatory shunts. I. Am Heart J 1951; 41:1-29
- HAKKI A, ABKULMASSIH IS. A simplified formula for the calculation of stenotic cardiac valves. Circulation 1981; 63:1050-5
- NISHIMURA RA, CARABELLO BA. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation 2012; 125:2138-50
- WINDECKER S, KPHL P, ALFONSO F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2014; 35:2541-619
