Evidence-based medicine
There is no definitive conclusion to be drawn from these remarks. We simply need to bear in mind the extreme complexity of the systems we observe, the relativity of our knowledge of them, and the fundamental distance that exists between conceptual thinking and reality. Our analysis of phenomena is based on their representation in our minds and remains dependent on the conventions of a language game. These are necessary for us to function, but they limit the scope of our knowledge.
In complex systems such as cardiac cell metabolism, the distribution of electrical depolarisation or coronary perfusion, it will never be possible to know exactly all the elements involved in the genesis of an event. What's more, it would not even be sufficient to predict its occurrence, which will remain random in our eyes. A complete understanding of all physiological processes is probably unattainable. In a sense, we have to give up trying to understand why a complex system like the heart behaves as it does at a given moment in its existence [10].
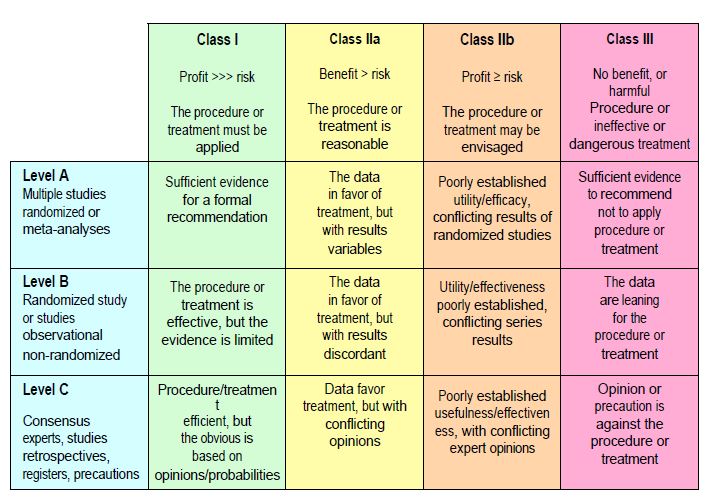
Despite this fundamental uncertainty, we need to be able to offer patients treatments that we have good reason to believe will work. To do this, we rely on evidence from a large number of randomised controlled trials (high level of evidence), or from a single randomised trial and/or observational series (moderate level of evidence). If this evidence is not available, we use expert opinion or the precautionary principle (low level of evidence). The level of evidence (levels A, B, and C) is therefore an assessment of the degree of certainty available on which to base a recommendation. The classes of recommendation (classes I, II and III) are an assessment of the benefits and risks of the proposed procedure or treatment (Figure 1.10).
- Level A: Data based on several large randomised controlled trials (RCTs) and/or their meta-analyses;
- Level B: Data based on a single randomised controlled trial or large prospective observational studies;
- Level C evidence: data based on retrospective studies, expert consensus or a precautionary principle;
- Class I recommendation: the benefits far outweigh the risks; the procedure or treatment is useful and effective; it should be used;
- Class IIa recommendation: the benefits probably outweigh the risks; the procedure or treatment is reasonable and should be performed;
- Class IIb recommendation: the benefit is doubtful; the procedure or treatment may be considered;
- Class III recommendation: the procedure or treatment is ineffective or dangerous and is contraindicated.
Figure 1.10: Classification of recommendations and levels of evidence, according to the system adopted by medical societies (American Society of Anesthesiology, Society of Cardiovascular Anesthesiologists, European Society of Anaesthesiology, American Heart Association, American College of Cardiology, European Society of Cardiology, Society of Thoracic Surgeons, etc). The classes of recommendations (classes I, IIa, IIb and III) are an estimate of the benefits and risks of the procedure or treatment envisaged. The level of evidence (levels A, B and C) is an assessment of the degree of certainty available on which to base a recommendation.
Since the work published by McMaster University (G. Guyatt, Hamilton, Canada) in the early 1990s, evidence-based medicine (EBM) has been considered the most scientific way to determine the validity of a therapy. It introduces a hierarchy of evidence, placing randomised controlled trials (RCTs) at the top of the list of reliable sources and clinical judgement and deductive reasoning at the bottom [1,9]. This position, which is widely shared by the medical establishment, nevertheless deserves a number of comments.
- Whether a piece of data seems conclusive depends on how it is interpreted. In the case of evidence-based medicine, this is the rational, mechanistic way of thinking of classical Western science. However reassuring it may be, the rigorous quantification of a phenomenon is an arbitrary and conventional breakdown of a fluid and moving reality into small independent units that can be manipulated mathematically.
- Conceptually, evidence is evidence only at the local level, in the event determined by the choice of population studied, the variables excluded and the question asked. This situation is very different from 'real life', where each element is embedded in a complex network of interactions and co-morbidities. Linear analysis of the behaviour of isolated fragments of a complex system cannot account for its emergent properties as an organised whole, because science is an attempt to simplify reality [7]. Although they rank lower than randomised trials in the hierarchy of sources, observational studies have the undeniable advantage of studying therapies in the real-life situation of patients.
- In the vast majority of randomised trials, the odds ratio between the treated group and the control group is between 0.5 and 0.8. The benefit of the intervention studied is therefore between 20% and 50%, which is still quite modest. Provided the trial is properly designed, this lack of power is due to the very concept of EBM. In a complex, multifactorial causality such as that of heart attack, it is inconceivable that changing just one causal factor could reverse the situation, because countless interactions and redundancies affect the outcome. The very nature of living organisms limits the scope of random assignment experiments.
- This way of understanding reality is not the only one available to the human mind. The concept of 'fuzzy logic' and the mathematics of chaos rehabilitate mechanisms such as intuition and clinical judgement, which analyse phenomena as a whole. These modalities, often described as subjective, are fundamental to our understanding of reality. We use them all the time when suggesting a treatment or choosing a salad. Rational reasoning is usually the justification for an option previously chosen at the subjective level. The belief in the primacy of quantitative evidence is a preconceived notion resulting from an unconditional and uncritical adherence to the deterministic paradigm of classical science: the claim to objectivity is in fact based on a non-objective first choice.
- Randomised controlled trials involving several thousand patients are undeniably statistically rigorous, but they are biased by the random and therefore indiscriminate distribution of the measure being evaluated within the chosen population. For example, the POISE trial found excess mortality associated with beta-blockers, but in patients who would never have received them because they were not indicated [4]. If the primary effect of a drug is useless, only its side effects will be seen. The risks associated with substances in situations where they have no indication lose their significance in situations where they are therapeutic. The same is true for monitoring techniques. Because of their broad coverage, RCTs are often at odds with targeted prospective studies that focus on the patients most likely to benefit from the substance.
- Between the lower threshold below which a variable is clearly normal and the upper threshold above which it is clearly pathological (or vice versa, depending on the variable chosen), there is always a zone of indeterminacy, often referred to as a 'grey area'. Depending on whether the focus is on specificity or sensitivity, the extent of the grey zone shifts towards its pathological or physiological end. Up to 25% of patients may be in this zone of undecidability, depending on the situation studied [3]. The fate of patients in this zone is a matter for the clinician's clinical judgement, not the evidence derived from an RCT.
- Between the lower threshold below which a variable is clearly normal and the upper threshold above which it is clearly pathological (or vice versa, depending on the variable chosen), there is always a zone of undecidability, often referred to as the 'grey zone'. Depending on whether the focus is on specificity or sensitivity, the extent of the grey zone shifts towards its pathological or physiological end. Up to 25% of patients may be in this zone of undecidability, depending on the situation studied [3]. The fate of patients in this zone is a matter for the clinician's clinical judgement, not the evidence derived from an RCT.
- Evidence-based medicine only provides an answer to a question that has already been asked. It does not allow you to explore a completely new field. It is blind to discoveries made by serendipity. Nor does it provide answers to situations that cannot be the subject of repeated trials, such as the identification of rare but very dangerous events. Giving priority to randomised controlled trials deprives medicine of original ideas and unexpected discoveries.
- The combination of EBM work and publication in peer-reviewed journals helps to shape a restrictive and dominant medical paradigm that tends to exclude any theory or approach that does not conform to conventional dogma. This is a far cry from the objectivity and neutrality to which this attitude claims to aspire.
- The results of RCTs make it possible to formulate recommendations (guidelines), which are a valuable aid to therapeutic decision-making, but which apply only to a specific population undergoing a specific intervention and which correspond only to predefined consequences (mortality, various morbidities) to the exclusion of all others (well-being, independence, mobility, etc.). However, as we have seen, almost half of the recommendations published in cardiology are not based on high-level trials (level A evidence), but on expert opinion, registries and retrospective studies (level C evidence) [11]. In a field as complex as medicine, they inevitably contain an element of uncertainty [5]. It is up to the clinical judgement of the practitioner to decide whether they apply to a particular patient. Applying recommendations to patients other than those in the original studies generally leads to poor outcomes [10].
- EBM provides a set of "objective" external data that must be combined with the clinician's expertise and the patient's contextual data (social, cultural, psychological, etc.). Effective patient management combines these three elements; it is not the simple application of a recommendation as if it were a legal requirement. A clinical protocol can never replace the clinician's judgement or the patient's experience.
- EBM gives more weight to statistical significance than to clinical significance, which is what matters to the patient. It applies to populations, not individuals. It focuses on the disease, not the patient. It develops a dogmatic attitude towards p < 0.05 and focuses on easily quantifiable markers such as mortality or the incidence of well-defined morbidities. It is reluctant to publish negative results and excludes older, polymorbid and non-compliant patients from its work, which distorts the purity of the results [9].
In contrast to EBM, 'personalised' or precision medicine is currently in full swing. This approach analyses the specific characteristics of individual patients (genomic variants, phenotypic constitution, addictions, psychosocial situation) and the impact of medical interventions on their personal future. Instead of studying the average results of a large but selected population, as EBM does, it takes into account the variable response of patients within a diagnostic group that is heterogeneous by nature [2]. Artificial intelligence and big data software are already making it possible to group patients according to phenotypic sets by analysing very large amounts of clinical, functional and therapeutic data [8]. This new paradigm will undoubtedly lead to more effective individualised care than the 'one size fits all' trend that still dominates EBM. In addition to the idea that each patient is different and requires tailored treatment, there is the fact that pathophysiological mechanisms are multifactorial and interdependent. There is no point in trying to solve a complex problem by focusing on a single factor. Similarly, the overall management of a patient requires a bundle of therapies acting at different points in the nosological cycle. Although it may seem trivial, this aspect of reality is largely overlooked by evidence-based medicine. Clearly, evidence-based medicine does not take all evidence into account.
Complexity and unpredictability
The occurrence of an emergent property in a complex system, such as a heart attack or ventricular tachycardia in a heart, is the result of so many possible combinations that predicting them poses unsolvable problems, both quantitatively and qualitatively. But a non-linear causal relationship is not a non-existent relationship. The absence of a direct relationship invites caution, not ignorance. For example, the absence of a clear relationship between intraoperative ischaemia seen on the ECG and postoperative infarction is no reason to ignore the phenomenon when it occurs. In clinical practice, prudence prevails: it is better to take unproven information seriously than to ignore a sign that might herald an accident. Our interventions in the human organism involve an incalculable number of processes, and their consequences are never fully predictable. As Stuart Kaufman suggests: "We can never be sure that our next step won't be the one that triggers the landslide of the century, so it's best to proceed with caution. We cannot know the true consequences of our actions. All we can do is act as best we can at the local level" [6]. Every detail of everyday life deserves the same care, not out of perfectionism but out of realism: in fact, any small gesture could be the one that causes the pile of sand to collapse, leading to a catastrophe. Complexity theories should therefore encourage us to be cautious and modest, as the next chapter will show (see Chapter 2, Safety and anaesthesia).
| Evidence-based medicine |
|
Evidence-based medicine (EBM) gives priority to the results of large-scale randomised controlled trials over observational studies, expert opinion or analysis of registries. - Level of evidence A: based on many large randomised controlled trials - Level B: single randomised controlled trial or large observational studies - Level C: retrospective studies, expert opinion, precautionary principle It derives clinical recommendations, which are divided into 4 categories - Class I: The procedure or treatment is useful and effective, and the benefit is clear. - Class IIa: the procedure or treatment is reasonable and should be used - Class IIb: the procedure or treatment can be considered, but the benefit is doubtful - Class III: the procedure or treatment is ineffective or dangerous (risk > benefit) However, this hierarchy of evidence suffers from certain weaknesses: priority of statistical significance over clinical significance, dependence on the rational and mechanistic way of thinking of classical science, validity limited to highly selected populations, ignorance of the weight of intuition and clinical judgement in medical decisions, ignorance of the socio-cultural and psychological context of the patient, extrapolation of recommendations derived from specific populations to the general population, limitation of consequences to quantifiable data (e.g. mortality) to the exclusion of qualitative aspects (e.g. well-being). While EBM is validated for populations, precision medicine focuses on individuals and aims to address the specific constellation of each person. Far from "one size fits all", anaesthesia techniques, monitoring and the dosage of drugs or infusions are adapted to the specific characteristics of each patient. |
© PG Chassot April 2007, last update September 2019
References
- ANDERSEN H. Mechanisms: what are their evidence for in evidence-based medicine? J Eval Clin Pract 2012; 18:992-9
- BLACKSTONE EH. Precision medicine versus evidence-based medicine. Individual treatment effect versus average treatment effect. Circulation 2019; 140:1236-8
- CANNESSON M, LE MANACH Y, HOFER CK, et al. Assessing the diagnostic accuracy of pulse pressure variations for the prediction of fluid responsiveness. A “grey zone” approach. Anesthesiology 2011; 115:231-41
- DEVEREAUX PJ, YANG H, YUSUF S, et al, for the POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE-trial): a randomised controlled trial. Lancet 2008; 371:1839-47
- IMBERGER G. Clinical guidelines and the question of uncertainty. Brit J Anaesth 2013; 111:700-2
- KAUFMAN S. At home in the Universe. The search for the laws of self-organisation and complexity. New York, Oxford University Press, 1995
- POPPER K. L'Univers irrésolu, plaidoyer pour l'indéterminisme. Hermann, Paris, 1984
- SHAH SJ, KITZMAN DW, BORLAUG BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation 2016; 134:73-90
- SHERIDAN DJ, JULIAN DG. Achievements and limitations of evidence-based medicine. J Am Coll Cardiol 2016; 68:204-13
- TINETTI ME, McAVAY G, TRENTALANGE M, et al. Association between guideline recommended drugs and death in older adults with multiple chronic conditions: population based cohort study. BMJ 2015; 351:h4984
- TRICOCI P, ALLEN JM, KRAMER JM, et al. Scientific evidence underlying the ACC/AHA clinical practice guidelines. JAMA 2009; 301:831-41
- ZWIRN HP. Les systèmes complexes. Mathématiques et biologie. Paris: Odile Jacob, 2006