Functional valve anatomy
Heart valve disease causes significant changes in preload, afterload, systolic performance and diastolic compliance. They induce ventricular remodelling and neurohumoral changes that are adaptive, but which progressively exceed the heart's ability to compensate. Left heart valve disease has an upstream effect on the pulmonary circulation, causing post-capillary hypertension. The four heart valves are located close together at the base of the heart. They are held together by the fibrous skeleton of the heart, which is made up of several components (Figure 11.1).
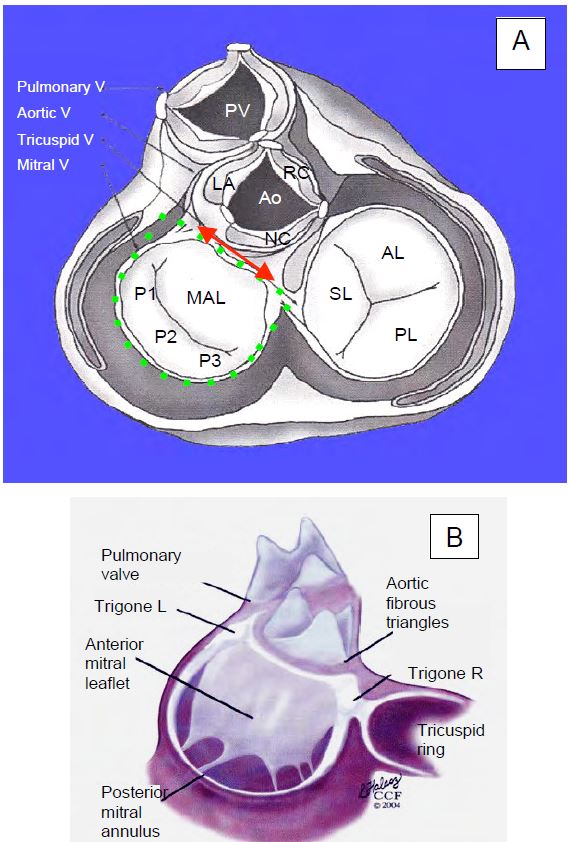
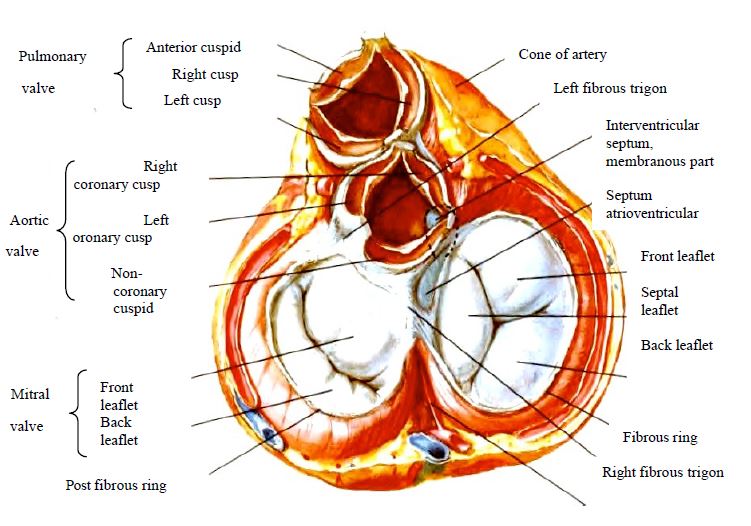
Figure 11.1: Anatomical diagram of the 4 heart valves seen from above after removal of the atria and arterial trunks. A: The mitral annulus has the approximate shape of an inverted letter 'D' (green dotted line), the base of which forms an angle of approximately 60° with the horizontal. PV: pulmonary valve. Ao: aortic valve. LC: left coronary cusp of the aortic valve. RC: right coronary cusp of the aortic valve. NC: non-coronary cusp of the aortic valve. MAL: anterior leaflet of the mitral valve. P1: anterior festoon of posterior mitral leaflet. P2: medial festoon of posterior mitral leaflet. P3: posterior festoon of the posterior mitral leaflet. SL: septal leaflet of the tricuspid valve. AL: anterior leaflet of the tricuspid valve. PL: posterior leaflet of the tricuspid valve. The double red arrow indicates the intertrigonal distance. B: Anatomical view of the mitral and tricuspid annuli; the tricuspid annulus is interrupted posterolaterally. The conduction bundle passes through the right trigone. The structure of the aortic valve is maintained by three fibrous triangles delimited by the insertion of the cusps on the wall of the sinuses of Valsalva [from Savage RM, Aronson S, Shernan SK. Comprehensive Textbook of Perioperative Transesophageal Echocardiography, 2nd Edition. Philadelphia: Wolters/Kluwer - Lippincott, Williams & Wilkins, 2011, Figure 30.6, p 492].
- The anterior (or left) trine, located between the mitral valve and the aortic valve.
- The posterior (or right) trine is located between the mitral valve, aortic valve and tricuspid valve; it overhangs the fibrous septum and is crossed by the conduction fascicles.
- The fibrous part that connects the two trigons onto which the mitral annulus is attached (base of the anterior leaflet).
- The fibrous mitral annulus, which becomes thinner in its posterior part opposite the medial scallop of the posterior leaflet.
- The fibrous structure of the aortic root supporting the 3 aortic cusps.
- The tricuspid annulus is thinner and interrupted in its posterolateral part.
The anterior pulmonary valve does not have an individualised fibrous ring; the right outlet chamber is entirely muscular.
Mitral valve
This name was given to the left atrioventricular valve by Vesalius in 1543 because of its resemblance to a bishop's mitre. However, the mitral valve is a dynamic structure made up of five different components (Figure 11.2) [4,7,10].
Video: Image of the two leaflets of the mitral valve in mid-oesophageal 5-cavity view; the anterior leaflet, which is longer, is on the left and the posterior leaflet on the right. A truncated view of the aortic valve appears on the far left of the screen.
Video: Front view of the mitral valve from the LA in three-dimensional reconstruction; the anterior leaflet is larger than the posterior leaflet; the commissure is C-shaped.
- A fibrous ring whose appearance, seen from the RA, resembles an inverted letter "D", the straight part of which is anterior, at the level of the fibrous trigone (area in telediastole: 5-6 cm2). The posterior part is thinner (see Figure 11.1B). In three dimensions, the mitral annulus has a saddle shape with the highest points anterior (aortic valve) and posterior; the lowest points are opposite the commissures. The normal AP diameter is 26-32 mm; the total height is approximately 10 mm (Figure 11.3) [16]. In telediastole, atrial contraction causes the annulus to narrow. In systole, the annulus undergoes 3 movements: its saddle shape increases, its circumference decreases by 25% and it slides approximately 1 cm towards the apex ("descent" of the mitral annulus) [11,18]. In the 4-cavity TEE view (0°), the annulus is sectioned in its lower part and the coaptation point appears in the plane of the annulus, whereas in the long-axis view (120°), the annulus is sectioned in its upper part and the coaptation point is located 5-7 mm below the plane of the annulus [6,14]. These concepts are important for the definition of mitral prolapse, which must be determined in the long-axis view (120-140°). Annular non-planarity is measured by the ratio of the height H (distance between the highest and lowest points, 8-10 mm) to the intercommissural distance (ICD, 30-35 mm), which is usually 0.2 [14]. Non-planarity is also assessed by the angle between the anterior and posterior points based on the intercommissural line; its normal value is > 130° [12]. These measurements are made on TEE using 3D reconstruction; some programmes calculate them automatically from geometric analysis of the annulus (Figure 11.4). As the LV dilates, the mitral annulus becomes more rounded and circular; it loses its saddle shape and flattens; the valve loses its seal in systole and the stress on the leaflets increases [5]. Dilatation occurs mainly at the expense of the posterior leaflet.
- Two leaflets; the posterior leaflet is arciform and divided into 3 anatomically well differentiated festoons (P1 anterior, P2 middle and P3 posterior); its insertion represents 2/3 of the circumference of the annulus. The anterior leaflet is more homogeneous and square in shape; its division into 3 parts A1, A2 and A3 does not correspond to distinct anatomical entities. Despite their different shapes, the two leaflets have an equivalent surface area. The length of the leaflets (distance from the base to the end of A2 or P2) is 22-23 mm for the anterior leaflet and 12-13 mm for the posterior leaflet.
-
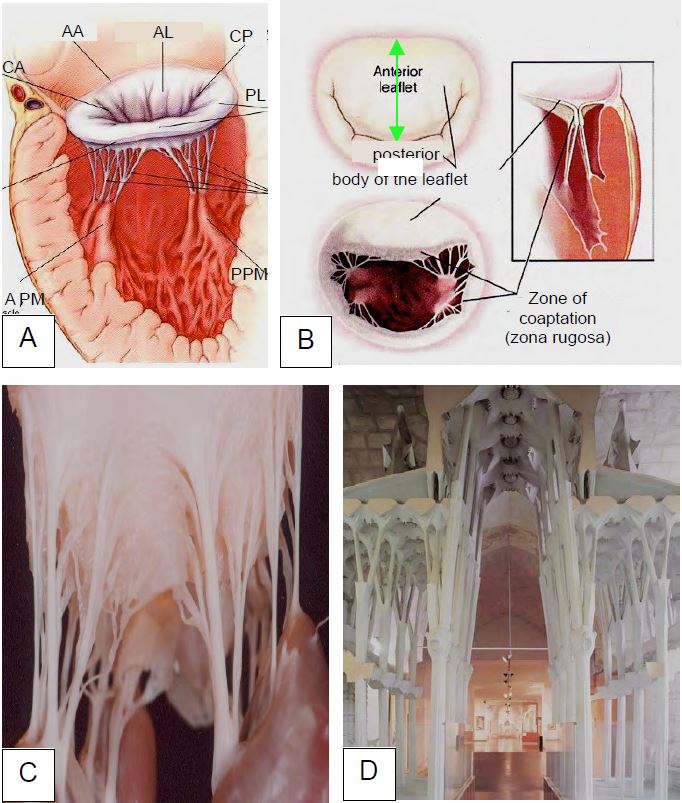
Figure 11.2: Overview of the mitral apparatus. A: The mitral valve with its ring, chords, papillary muscles and ventricular wall. B: The anterior leaflet is "square", whereas the posterior leaflet is crescent-shaped; the pillars are perpendicular to the commissures; on occlusion, intraventricular systolic pressure presses the free edges of the two leaflets together along the zona rugosa; the height of coaptation is 0.6-1.0 cm. The length of the leaflets (green arrow) is 22-23 mm for the anterior leaflet and 12-13 mm for the posterior leaflet [4]. C: Intraventricular view of the subvalvular apparatus anchored to the pillars; this structure resembles the pillars and vaults of a cathedral (D: Sagrada Familia, Antoni Gaudi, Barcelona) because they have the same function: to support pressure. AA: anterior ring (fibrous trigone). AL: anterior leaflet. AC: Anterior commissure. PC: posterior commissure. PL: posterior leaflet. APM: anterior papillary muscle. PPM: posterior papillary muscle.
-
25-30 cords inserted on the ventricular face of the leaflets; 1st order cords are attached to the distal end and 2nd order cords to the body of the leaflets; 3rd order cords are attached near the base and maintain the geometry of the ventricle; cords are on average 1.5 cm long.
- Two papillary muscles, one antero-lateral and the other postero-medial, located vertically above the commissures; the bundle of cords implanted on each pillar is distributed over both layers, either anteriorly (antero-lateral pillar ALP) or posteriorly (postero-medial pillar PMP). The ALP is vascularised by two coronary networks (IVA and CX), whereas the PMP is only vascularised by the RC. The PMP is often bifid.
-
The wall of the LV on which each abutment is implanted; contraction of this wall is essential to ensure mitral occlusion; in the event of akinesia or dilation, the radial shortening defect maintains excessive traction on the corresponding chords and prevents the leaflets from reaching their coaptation point (see Figure 11.53).
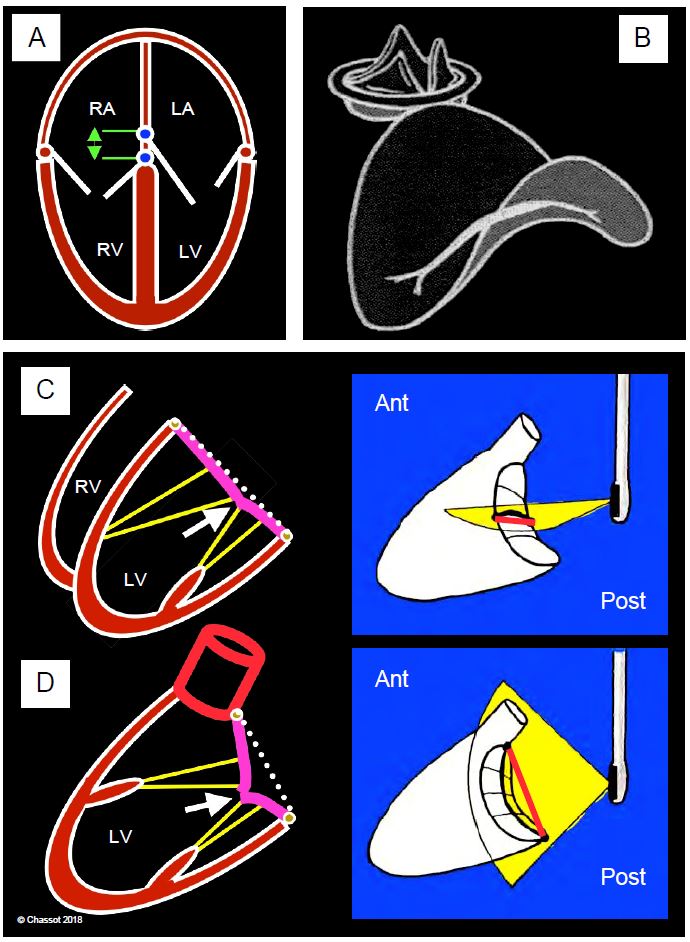
Figure 11.3: Mitral annulus configuration. A: The septal insertion of the mitral valve is more proximal than that of the tricuspid valve, which is more apical; the offset is 1 cm (green). B: Saddle shape of the mitral annulus, with the highest points anterior (aortic valve) and posterior. C: In the 4-cavity view, the annulus is sectioned at its lowest points; the coaptation point of the mitral valve (white arrow) appears in the plane of the annulus. D: In the long-axis view, the annulus is sectioned at its highest points; the coaptation point of the mitral valve appears below the plane of the annulus. This difference between the two views is important in the definition of mitral prolapse [1].
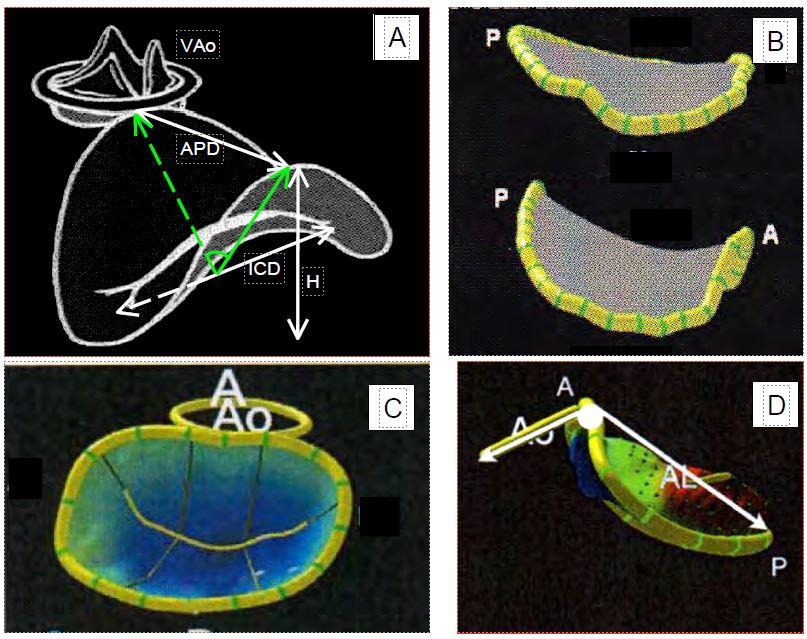
Figure 11.4: Saddle shape of the mitral annulus in three-dimensional reconstruction. A: The highest points are anterior (aortic annulus) and posterior and define the anteroposterior diameter (APD); the lowest points are opposite the valve commissures and define the second valve diameter. The height of the annulus (H) is approximately 10 mm; the intercommissural distance (ICD) runs from one commissure to the other. The angle of elevation (green) is based on the intercommissural line and defines the opening between the two highest points. B: Compared to diastole (top), the saddle shape becomes more pronounced in systole (bottom). C and D: 3D parametric echo reconstruction images (Q-Lab™ programme, Philips) of the mitral and aortic annuli in two pathological situations. C: Restriction of leaflet trajectory due to ventricular dilatation; the blue colouring indicates the area below the plane of the annulus. D: Prolapse of both leaflets in Barlow's disease, with determination of the mitral angle between the planes of the two valves. A: Anterior. P: Posterior.
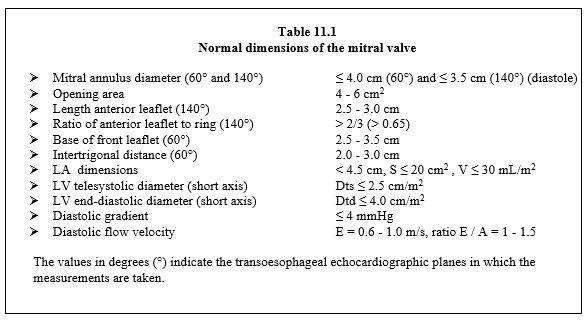
In diastole, the mitral valve has an open area of 4-6 cm2. Its gradient is ≤ 4 mmHg and may increase if systolic volume is greatly increased (mitral regurgitation). In systole, the mitral valve is closed by two opposing forces (Figure 11.5).
- Occluding force: intraventricular pressure (100-150 mmHg) forces the free edges of the two leaflets together along the zona rugosa; the height of this coaptation surface is 0.6-1.0 cm and represents 20-25% of the total leaflet length.
- Tethering force: Contraction of the ventricular wall and papillary muscles tensions the chords, holding the valve in its coaptation plane and preventing it from tilting into the LA.
If the mitral leaflets are occluded edge-to-edge, the valve cannot withstand the intraventricular pressure; it loses its seal and becomes inadequate. The normal dimensions of the mitral valve are given in Table 11.1 [13,17].
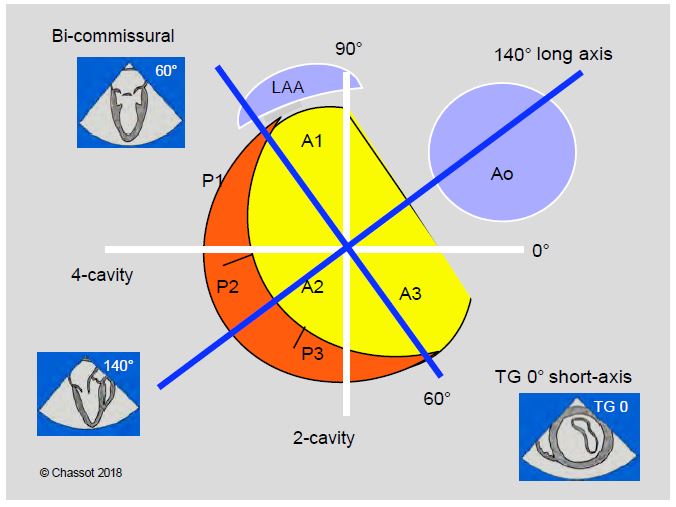
The orthogonal planes for the mitral valve on TEE are different from the orthogonal planes of the heart. These are the horizontal 4-cavity 0° plane and the sagittal 2-cavity 90° plane. However, the mitral valve rotates about 60° in the frontal plane relative to the body (Figures 11.6). Its orthogonal planes are therefore the 40-60° bi-commissural plane, which cuts it through the two commissures, and the 120-150° long-axis plane, which cuts it through the greatest length of the leaflets (A2 and P2); this plane includes the long-axis view of the left ventricle and the aortic valve; it is approximately perpendicular to the previous plane (Figure 11.7). To these two planes is added the short axis 0-20° transgastric view of the mitral valve, but this view is not always feasible.
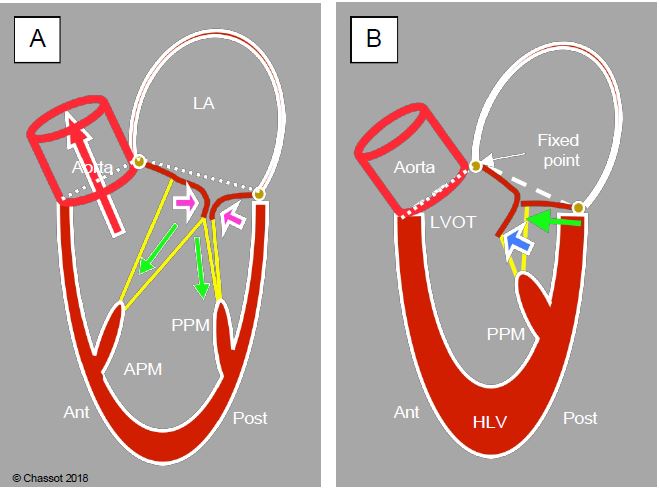
Figure 11.5: LV contraction. A: The increase in intraventricular pressure during systole forces the two mitral leaflets together along their free edges (purple arrows); contraction of the wall and papillary muscles (APM: anterior papillary muscle, PPM: posterior papillary muscle) tightens the chords and maintains the mitral valve in its closed plane (green arrows). The balance of these two forces forms the internal skeleton of the ventricle, which allows blood to be ejected into the LV outflow tract (LVOT) without the ventricle twisting on itself. The contraction starts at the apex and ends in the plenum. B: If the course of the posterior wall and the PPM is too long or if the ventricle is too small in systole (catecholaminergic overstimulation, hypovolaemia, concentric hypertrophy), the posterior wall moves anteriorly (green arrow); the coaptation point of the mitral valve is displaced anteriorly towards the aorta and approaches the LVOT; the tip of the posterior leaflet is in contact with the body of the anterior leaflet. The anterior leaflet is then pushed towards the LVOT as intraventricular pressure increases during systole (blue arrow). White line: mitral valve angle.
Figure 11.6: TEE cross sections of the mitral valve. The orthogonal cross-sectional planes of the body (4-cavity 0° and 2-cavity 90°, in white) do not correspond to the orientation of the mitral valve. The mitral valve is off-axis by 60°, so its orthogonal planes (in blue) are the bi-commissural plane at 40-60° and the long-axis plane at 120-150°, which is perpendicular to it and intersects the chase chamber and the aortic valve. The left atrial appendage (LAA) lies opposite the anterior mitral commissure. The third orthogonal plane is the 0° transgastric short axis view.
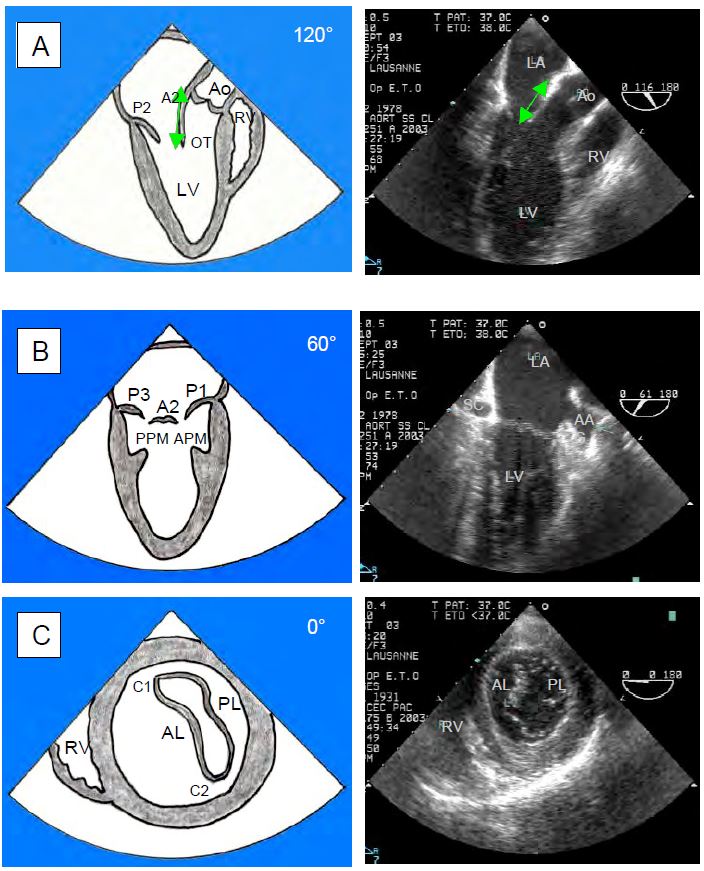
Figure 11.7: Transesophageal echocardiographic views of the mitral valve. These are orthogonal views of the valve. A: 120° long-axis view of the LV with measurement of the length of the anterior leaflet at A2. B: 60° bi-commissural view. C: 0° transgastric short axis view. OT: Outflow tract. APM: anterior papillary muscle. PPM: Posterior papillary muscle. C1: Anterior commissure. C2: posterior commissure. The diameter of the mitral annulus is measured at 120-140° (A) and 60° (B) [1].
Aortic valve
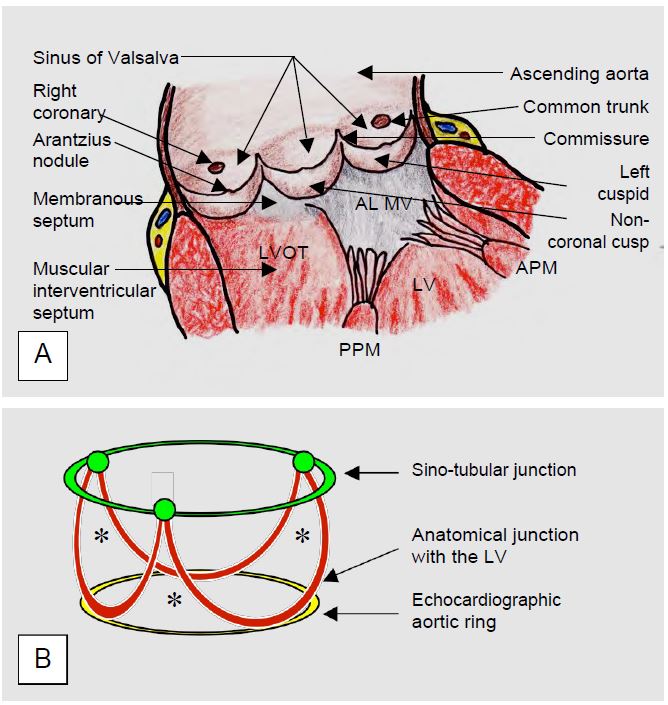
The aortic valve is part of the dynamic assembly that forms the root of the aorta. This assembly consists of several components (Figures 11.8) [1,19].
- A crown-shaped fibrous aortic structure with 3 vertical branches arising from the cusp commissures. The junction between the musculature of the LV outflow tract and the fibrous tissue of the aorta is incorrectly referred to as the aortic 'ring', although there is no separate anatomical structure at this level.
- Three semilunar cusps defined by their body, their free edge and their U-shaped base on the aortic wall opposite each sinus of Valsalva; the base measures 1.5 times the free edge. The lower insertion of the cusps is on the basal ring, which is anatomically located in the LV outflow tract (LVOT), below the junction between the aorta and the LV. The cusps are attached to the sinotubular junction.
- Three sinuses of Valsalva, which are bulges in the aortic root opposite the cusps; the right coronary sinus arises from the right sinus (the most anterior); the common trunk arises from the left sinus; the non-coronary sinus is the most posterior.
- Sinotubular junction between the sinuses of Valsalva and the ascending aorta, where the commissures of the valve remain.
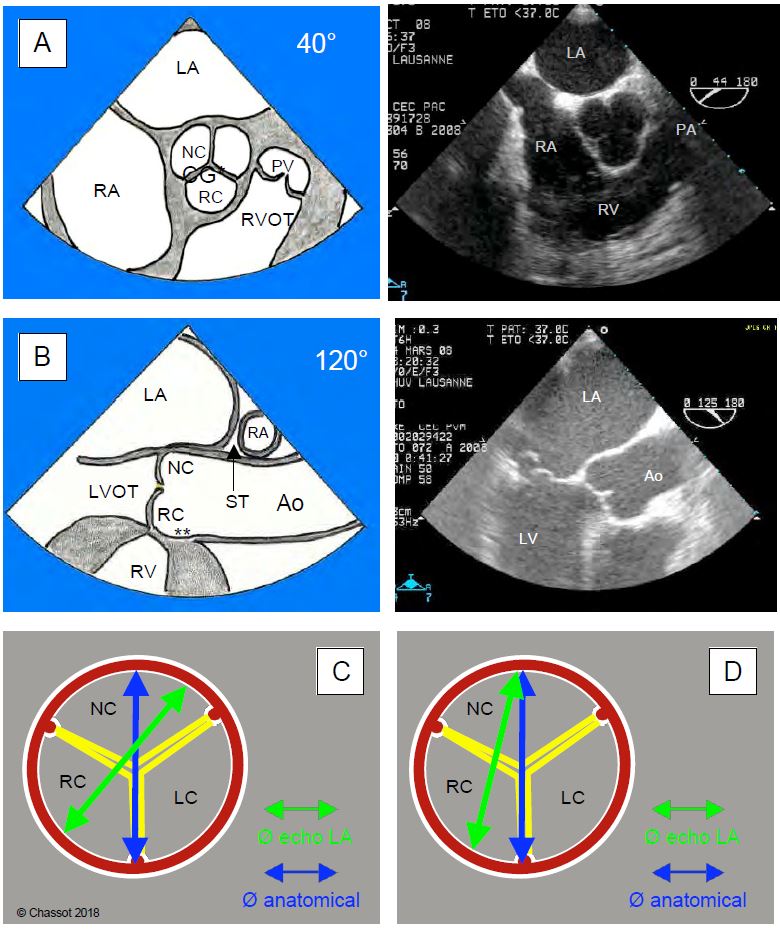
The aortic valve lies anterior to the mitral valve, oriented so that the non-coronary (NC) leaflet is posterior, opposite the interatrial septum; the right coronary (RC) cusp is the most anterior (Figure 11.9). The height of each leaflet is 13-15 mm, but their surface is slightly asymmetrical: the NC leaflet is slightly larger than the right and left coronary leaflets. The central part of the free edges shows a fibrous reinforcement corresponding to their coaptation point (Arantius nodules); on the ventricular face of the cusps there is fibrous degeneration (Lambl excrescences).
Video: Short-axis view of the aortic valve (40° mid-oesophageal view) with its three cusps; the non-coronary cusp is at 10 o'clock, opposite the interatrial septum; the right coronary cusp is at 6 o'clock and the left coronary cusp is at 2 o'clock.
Video: Long-axis view of the aortic valve (120° mid-oesophageal view); the cusp towards the top of the screen is the non-coronary, the cusp towards the bottom is the right coronary. The mitral valve shows the central part of the anterior leaflet (A2, longer) and the central part of the posterior leaflet (P2, shorter).
The aortic valve overhangs the left ventricle, the walls of which are formed by the anterior leaflet of the mitral valve, the interventricular septum and the posterior wall of the LV. The two left valves are contiguous: the base of the anterior mitral leaflet lies on the fibrous trigone opposite the left aortic and non-coronary leaflets (see Figure 11.1).
Figure 11.8: The aortic valve. A: Aortic valve and its anatomical connections. FLMV: Frontal leaflet of the mitral valve. APM: anterior papillary muscle. PPM: posterior papillary muscle. LVOT: left ventricular outflow tract. B: Schematic representation of the structures of the aortic valve; the base of the cusps (in brown) is U-shaped; the aortic ring (in yellow) visible on echocardiography is slightly narrower than the anatomical junction between the aortic structure and the LV; it is also slightly narrower than the sino-tubular junction (in green). *Approximately triangular spaces which are the fibrous structure supporting the aortic valve [19].
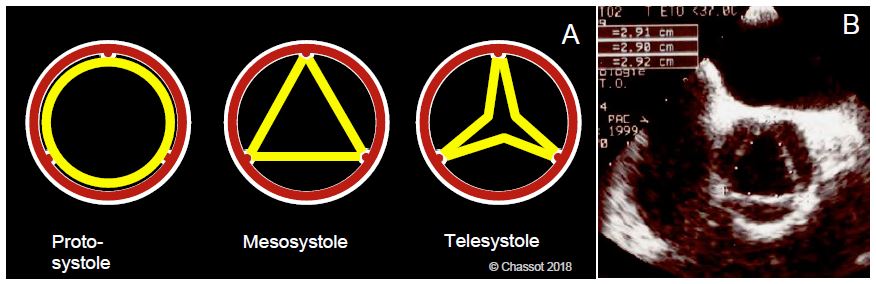
In systole, the opening of the aortic valve is 3 - 4 cm2 , but the shape of this opening changes during ejection (Figure 11.10) [2].
- Circular in protodiastole, during peak flow (maximum velocity);
- Triangular for ≥ 70% of ejection time;
- 3-pointed star in telesystole, when flow becomes weak.
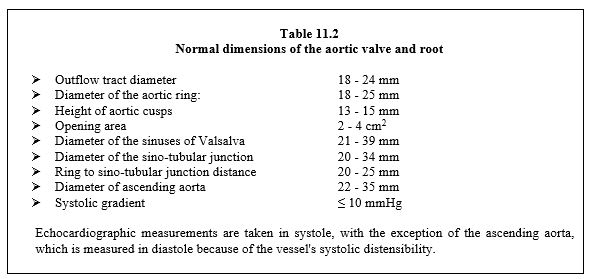
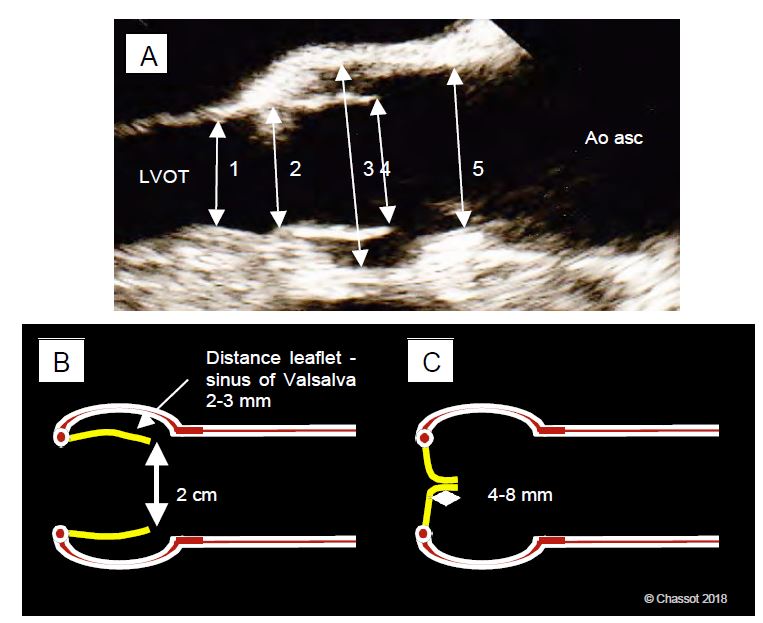
The position of the open cusps is practically parallel to the blood flow during ejection; the flow remains laminar (velocity 1.0 - 1.5 m/s) and the gradient is < 10 mmHg because the diameter between the outflow chamber, the aortic ring, the cusps and the root of the ascending aorta changes very little. The free edge of the cusps is 2.0-2.2 cm apart. The sinuses of Valsalva protrude 2-3 mm from the aortic annulus. Their presence prevents the cusps from adhering to the aortic wall during systole (Figure 11.11). If they were to adhere to the wall during systole, the cusps might not close immediately during protodiastole; this delay would lead to constant aortic leakage. In diastole, the height of coaptation of the cusps by their free edge is 4-8 mm; it is the diastolic aortic pressure that provides the seal by pressing the cusps together. The normal dimensions of the aortic valve and root are given in Table 11.2 [3,13,17].
Figure 11.9: TEE views of the aortic valve. A: 40° short-axis basal mid-oesophageal view. B: 120° long-axis view of the aortic root. NC: non-coronal cusp. LC: left coronary cusp. RC: right coronary cusp. PV: pulmonary valve. RVOT: RV outflow tract. *: Departure from the common trunk. **: Departure from the right coronary artery [1]. C: The diameter of the aortic annulus measured in the 120° long-axis view (Ø echo LA, in green) on TEE is slightly narrower than the anatomical diameter (in blue) because it does not pass through the centre of the annulus. D: In the parasternal transthoracic long-axis view (TTV), the difference is much more pronounced [19].
Figure 11.10: Aortic valve opening in systole. A: Its shape and surface area change during ejection. B: Dimensions of the aortic valve in its isosceles triangular mesosystolic shape; the dimensions shown are the lengths of each side. The triangular shape is suitable for measuring stroke volume (SV = orifice area x integral of velocities) because it is the shape of the valve during most of systole [2].
Figure 11.11: Dimensions of the aortic valve and root. A: 120° long axis diameter (TEE). 1: LV outflow tract; 2: aortic annulus; 3: sinus of Valsalva; 4: systolic leaflet spacing; 5: sinotubular junction; these diameters are measured in systole. The diameter of the ascending aorta is measured in diastole at the level of the crossing of the right pulmonary artery (not visible in the image). B: Distance between the cusps in systole; they are not attached to the wall of the sinuses of Valsalva, but are 2-3 mm apart. The lumen - aortic annulus - cusps - sinotubular junction - ascending aorta is practically a tube of constant diameter; the flow is laminar. C: In diastole, the coaptation height of the free edge of the cusps is 4-8 mm; it is the diastolic pressure of the aorta that keeps them closed.
Tricuspid valve
The tricuspid valve is attached to an incomplete horseshoe-shaped fibrous ring centred on the right trigone and interrupted posterolaterally. At the level of the septum, this ring is approximately 1 cm more apical than the mitral ring (Figure 11.12). It is also saddle-shaped and approximately ovoid in shape, with the long axis running anteroposteriorly and the base widening in the posterior leaflet. Its anatomical diameter is 3 - 4 cm; in 4-cavity view the normal diameter is 2.8 ± 0.5 cm [8,9]. The surface area of the annulus contracts by approximately 30% during systole [15]. As the name suggests, the valve is composed of three leaflets, which are not equal in size.
- The anterior leaflet is the largest and most mobile;
- Posterior leaflet;
- Septal leaflet, the smallest and most restrictive.
Figure 11.12: The tricuspid valve (TrV) in anatomical position with its three leaflets: anterior (largest), posterior and septal (smallest). The general shape of the valve is elliptical with a long antero-posterior axis. At the level of the septum (atrioventricular septum), the tricuspid annulus is about 1 cm lower than the mitral annulus. MV: Mitral valve.
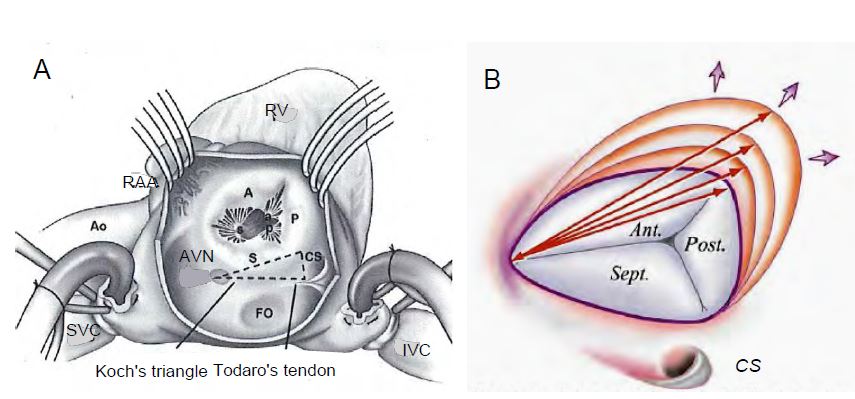
These leaflets are thin (< 1 mm thick) and arachnoid; their commissures are difficult to see on echocardiography. The valve is connected to 3 pillars, one of which is located on the septum; the anterior pillar, the largest, is located at the origin of the RV moderator band (muscular trabeculae that connect the septum to the anterior wall of the ventricle). The anteroseptal commissure is 3-5 mm from the AV node and His bundle; the posteroseptal commissure is opposite the coronary sinus outlet (Figure 11.13). In right-sided overload, the valve dilates latero-posteriorly where its annulus is interrupted. The tricuspid valve is part of a low-pressure system (the systolic pressure of the RV is six times lower than that of the LV). As a result, it does not need to be completely leak-proof; a small tricuspid leak occurs in >50% of the normal population. Normal tricuspid valve values are summarised in Table 11.3 [13,17].
Figure 11.13: Tricuspid valve. A: Surgical view from a RA atriotomy (lateral surgical approach). The 3 leaflets (anterior A, posterior P and septal S) are unequal in size: A > P > S. The septal insertion is bordered by Koch's triangle (AV node - Todaro's tendon - coronary sinus), which contains the conduction bundles. AVN: Atrioventricular node. CS: coronary sinus. FO: Fossa ovale. RAA: right atrial appendage [15]. B: As its annulus dilates, the tricuspid valve enlarges in a lateral-posterior direction at the level of the anterior and posterior leaflets. This image also illustrates the ovoid shape of the valve with its long anteroposterior axis and wider base in the posterior leaflet.
Pulmonary valve
The pulmonary valve is often involved in congenital diseases, but is rarely affected in adults. It lies in front of the aortic valve, has no fibrous ring (the right outlet chamber is entirely muscular) and has three leaflets: right, left and anterior, mirror images of those of the aortic valve (Figure 11.1). Its normal surface area is 2 cm /m2 . It has a small leak in > 50% of the normal population.
| Heart valves anatomy |
|
The two atrioventricular valves are posterior to the aortic valve; the pulmonary valve is the most anterior.
Mitral valve: approximately square anterior leaflet and crescent-shaped posterior leaflet (divided into 3 festoons). Planes orthogonal to TEE: bicommissural view 40-60° and long-axis view 120-140°.
Aortic valve: 3 cusps (right coronary, left coronary and non-coronary). TEE slice planes: Mid-oesophagus view short axis 40° and long axis 120-130°. Tricuspid valve: 3 leaflets (anterior, posterior and septal). Pulmonary valve: 3 leaflets (right, left and anterior). |
© CHASSOT PG, BETTEX D, August 2011, last update November 2019
References
- BETTEX D, CHASSOT PG. Transoesophageal echocardiography in anaesthesia and intensive care. Paris: Masson, Williams & Wilkins, 1997, 454 pp.
- DARMON PL, HILLEL Z, MOGTADER A, et al. Cardiac output by transesophageal echocardiography using continuous-wave Doppler across the aortic valve. Anesthesiology 1994; 80:796-805
- FAN CM, LIU X, PANIDIS JP, et al. Prediction of homograft aortic valve size by transthoracic and transesophageal two-dimensional echocardiography. Echocardiography 1997; 14:345-8
- FISCHER GW, ANYANWU AC, ADAMS DH. Intraoperative classification of mitral valve dysfunction: The role of the anesthesiologist in mitral valve reconstruction. J Cardiothorac Vasc Anesth 2009; 23:531-43
- GLASSON JR, KOMEDA M, DAUGHTERS GT, et al. Three-dimensional dynamics of the canine mitral annulus during ischemic mitral regurgitation. Ann Thorac Surg 1996; 62:1059-68
- GREWAL J, MANKAD S, FREEMAN WK. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009; 22:34-41
- LAMBERT AS, MILLER JP, MERRICK SH, et al. Improved evaluation of the location and mechanism of mitral valve regurgutation with a systematic transesophageal echocardiography examination. Anesth Analg 1999; 88:1205-12
- LANCELLOTTI P, MOURA L, AGRICOLA E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010; 11:307-32
- LANCELLOTTI P, TRIBOUILLOY C, HAGENDORFF A, et al. Recommendations for the echocardiographic assessment of native valvular regurugitation: an executive summary from the EACI. Eur Heart J Cardiovasc Imaging 2013; 14:611-44
- LEVINE RA, HAGÉGE AA, JUDGE DP, et al. Mitral valve disease - morphology and mechanisms. Nat Rev Cardiol 2015; 12:689-710
- MAHMOOD F, MATYAL R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg 2015; 121:34-58
- MAHMOOD F, SHAKIL O, MAHMOOD B, et al. Mitral annulus: an intraoperative echocardiographic perspective. J Cardiothorac Vasc Anesth 2013; 27:1355-63
- OH JK, SEWARD JB, TAJIK AJ. The echo manual. 3rd edition. Philadelphia, Lippincott Williams & Wilkins, 2006, 189-242
- POUCH AM, VERGNAT M, McGARVEY JR, et al. Statistical assessment of normal mitral annular geometry using automated three-dimensional echocardiographic analysis. Ann Thorac Surg 2014; 91:71-7
- ROGERS JH, BOLLING SF. The tricuspid valve. Current perspective and evolving management of tricuspid regurgitation. Circulation 2009; 119:2718-25
- SALGO IS, GORMAN JH, GORMAN RC, et al. Effect of annular shape on leaflet curvature in reducing mitral stress. Circulation 2002; 106:711-7
- SAVAGE RM, ARONSON S, SHERNAN SK. Comprehensive textbook of perioperative transesophageal echocardiography, 2nd edition. Philadelphia: Wolters/Kluwer - Lippincott, Williams & Wilkins, 2011,
- YAMAURA Y, YOSHIKAWA J, YOSHIDA K, et al. Three-dimensional analysis of configuration and dynamics in patients with an annuloplasty ring by multiplane transesophageal echocardiography: comparison between flexible and rigid annuloplasty rings. J Heart Valve Dis 1995;4:618-22
- ZAMORANO JL, BADANO LP, BRUCE C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur J Echocardiogr 2011; 12:557-84